Nuclear Fission
Nuclear fission of heavy elements was discovered in 1938 by German Otto Hahn and Fritz Strassmann. Nuclear fission is a nuclear reaction or a radioactive decay process in which the nucleus of an atom splits into two or more smaller, lighter nuclei. This process usually produces free neutrons, gamma photons and releases a very large amount of energy as per standards of radioactive decay.
In a nuclear reactor, a neutron is absorbed into a nucleus usually uranium-235, this makes the nucleus to become uranium 236, which is violently unstable. The nuclear fission reaction is normally a chain reaction, what this means is that, the entire nucleus split into two large particles, commonly referred to as ‘’daughter nuclei’’. In addition to the ‘daughter’ nuclei, two or three neutrons explode out of the fission reaction and they eventually collide with other uranium nuclei to cause further fission reaction.
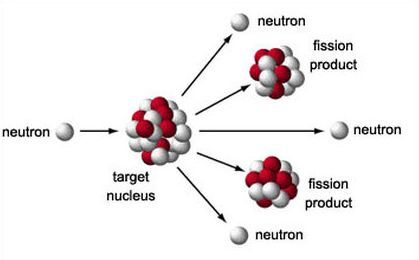
In Nuclear Chemistry or Physics, iron (Fe) is theoretically considered as a boundary due to its highest binding ability energy per nucleon. Therefore, all elements in the periodic table after iron (Fe) have high probability of undergoing nuclear fission. Generally, the conclusion is always that, fission is highly possible for atoms with higher atomic number.
Common radio isotopes that are used as fuel in nuclear reactors to induce fission are Uranium-233, Thorium-232, Uranium-235 and plutonium-239. Nuclear fission is used to generate power referred to as nuclear power. In this case, uranium-235 is used as the nuclear fuel and its fission is triggered by the absorption of a slow moving thermal neutron.
In our modern world, fusion has proved to be extremely expensive because it generates huge amounts of radioactive wastes and raises fundamental concerns about safety and proliferation of weapons.
What You Need To Know About Nuclear Fission
- Nuclear fission was first discovered by scientist Otto Hahn and Fritz Strassmann in 1938.
- Nuclear fusion is the breakdown of a heavy nucleus into two lighter nuclei due to bombardment of neutron.
- Nuclear fission generates a lot of radioactive particles.
- Nuclear fission reactor is based on a concept of controlled fission chain reaction.
- Fission is induced by neutrons.
- Common radio isotopes that are used as fuel in fission nuclear reactors are Uranium-233, Uranium-235 and plutonium-239.
- In nuclear chemistry or physics, iron (Fe) is theoretically considered as a boundary due to its highest binding ability energy per nucleon. Therefore, all elements in the periodic table after iron (Fe) have high probability of undergoing nuclear fission. Generally, the conclusion is always that, fission is highly possible for atoms with higher atomic number.
- Nuclear fission can undergo spontaneous chain reaction i.e one fission reaction automatically influences another fission reaction.
- Nuclear fission chain reaction can be controlled and manipulated effectively for other uses.
- To completely initiate a fission reaction a high velocity neutron is required together with a critical mass of fuel to sustain the chain reaction.
- Fission offers low energy density because energy released per unit nucleon is relatively lower.
- Fuel used in fission reactors is either in a solid or liquid state.
- Uncontrolled fission reaction (spontaneous chain reaction) results to atomic bomb.
- Fission is a single stage reaction.
- Nuclear fission is employed in nuclear reactors in power plants for generation of electricity.
Nuclear Fusion
Nuclear fusion is reaction in which two or more atomic nuclei are combined to form one or more different atomic nuclei and subatomic particles (neutrons or protons). In the 1930’s researchers, particularly Hans Bethe discovered that nuclear fusion was possible and that it was the energy source for the sun.
Nuclear fusion is achieved by combining two isotopes of hydrogen: deuterium (H-2) and tritium (H-3) in high density, high temperature environment. In a fusion cycle, tritium (H-3) and deuteriumH-2) are combined and result in the formation of helium (among the heaviest element in the periodic table), and the release of a free neutron. Theoretically in nuclear chemistry or physics, all elements in the periodic table before iron (Fe) have high probability of undergoing nuclear fusion. Generally, the conclusion is always that, Fusion is highly possible for elements with lower atomic numbers.
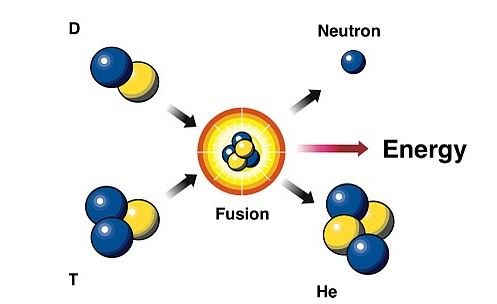
Generally, one good thing about nuclear fusion is that it does not generate radioactive particles and produces large amounts of energy. However, depending on the radio isotopes used as fuel, a few radioactive particles can be generated.
Research into developing controlled fusion inside fusion reactors has been ongoing since 1940s. This research is in an effort to make a fusion reactor to produce electricity. Some researchers still believes that there are opportunities with such a power source since fusion generates less radioactive material than fission and has nearly unlimited fuel supply. However, progress is slow due to challenges with understanding how to control the reaction in a contained space.
What You Need To Know About Nuclear Fusion
- Nuclear fusion was first discovered by scientist Hans Bethe 1932.
- Nuclear fusion is the combination of two lighter nuclei to form a heavy nucleus.
- Nuclear fusion generally does not generate radioactive particles. However, depending on the radio isotopes used as fuel, a few radioactive particles can be generated.
- Controlled fusion reaction is yet to be discovered.
- Fusion is induced by protons.
- Common isotopes of hydrogen that are used as fuel in fusion nuclear reactors are Deuterium (H-2) and tritium (H-3). Though as of now, the two isotopes (H-2 and H-3) are usually used for trial and research purposes only.
- Theoretically all elements in the periodic table before iron (Fe) have high probability of undergoing nuclear fusion. Generally, the conclusion is always that, Fusion is highly possible for elements with lower atomic numbers.
- Nuclear fusion has no ability to undergo a spontaneous chain reaction i.e one fusion reaction does not influence another fusion reaction.
- It is highly impossible to control a nuclear fusion reaction after its initiation.
- Three conditions are generally critical to initiate a fusion reaction; they include critical ion density, sufficient confinement time and very high temperature in the order of 107 to 108 Kelvin.
- Fusion offers high energy density because energy released per unit nucleon is significantly higher.
- Fuel used in fusion reactors is in plasma state.
- Uncontrolled fusion reaction results in a hydrogen bomb which is relatively 1000 times powerful than the atom bombing.
- Fusion is a multi-stage reaction.
- Nuclear fusion occurs in the sun and other stars.
Difference Between Nuclear Fission And Nuclear Fusion In Tabular Form
| BASIS OF COMPARISON | NUCLEAR FISSION | NUCLEAR FUSION |
| Discovery | Nuclear fission was first discovered by scientist Otto Hahn and Fritz Strassmann. | Nuclear fusion was first discovered by scientist Hans Bethe. |
| Description | Nuclear fusion is the breakdown of a heavy nucleus into two lighter nuclei due to bombardment of neutron. | Nuclear fusion is the combination of two lighter nuclei to form a heavy nucleus. |
| Radioactive Particles | Generates a lot of radioactive particles. | Generally does not generate radioactive particles. However, depending on the radio isotopes used as fuel, a few radioactive particles can be generated. |
| Control | Fission reactor is based on a concept of controlled fission chain reaction. | Controlled fusion reaction is yet to be discovered. |
| Inducement | Fission is induced by neutrons. | Fusion is induced by protons. |
| Radio Isotopes Used As Fuel | Common radio isotopes that are used as fuel in fission nuclear reactors are Uranium-233, Uranium-235 and plutonium-239. | Common isotopes of hydrogen that are used as fuel in fusion nuclear reactors are Deuterium (H-2) and tritium (H-3). |
| Atom/Elements That Can Make Reaction Possible | Fission is highly possible for atoms with higher atomic number. | Fusion is highly possible for elements with lower atomic numbers. |
| Chain Reaction | Can undergo spontaneous chain reaction. | Has no ability to undergo a spontaneous chain reaction. |
| Control After Initiation | Chain reaction can be controlled and manipulated effectively for other uses. | It is highly impossible to control a nuclear fusion reaction after its initiation. |
| Mandatory Condition For Reaction To Occur | A high velocity neutron is required together with a critical mass of fuel to sustain the chain reaction. | Critical ion density, Sufficient confinement time and very high temperature in the order of 107 to 108 Kelvin. |
| Energy Released | It offers low energy density because energy released per unit nucleon is relatively lower. | It offers high energy density because energy released per unit nucleon is significantly higher. |
| State Of Fuel Used | Fuel used in fission reactors is either in a solid or liquid state. | Fuel used in fusion reactors is in plasma state. |
| Effect Of Uncontrolled Reaction | Uncontrolled fission reaction (spontaneous chain reaction) results to atomic bomb. | Uncontrolled fusion reaction results in a hydrogen bomb which is relatively 1000 times powerful than the atom bombing. |
| Type Of Reaction | Fission is a single stage reaction. | Fusion is a multi-stage reaction. |
| Use | Nuclear fission is employed in nuclear reactors in power plants for generation of electricity. | Nuclear fusion occurs in the sun and other stars. |