Polymers are linearly chained large molecules composed of sequences of repeating monomer units connected by covalent bonds. Polymers can be classified as organic or inorganic polymers. Polymer as a chemical compound has high molecular weight consisting of a number of structural units linked together by covalent bonds. (A structural unit is a group having two or more bonding sites). Polymers containing inorganic and organic components are sometimes called hybrid polymers. Let’s look at the difference between the two.
Also Read: Difference Between Organic And Inorganic Compounds
Organic Polymers
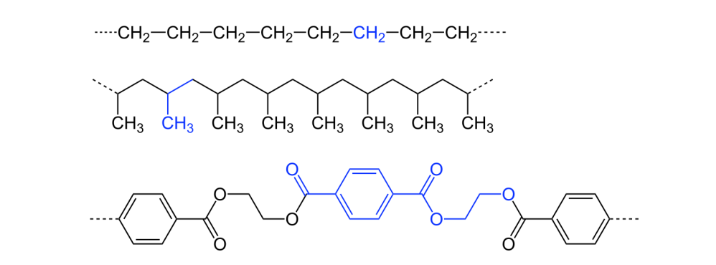
An organic polymer is a large molecule composed primarily of carbon atoms, along with hydrogen, oxygen, nitrogen, and other elements. These polymers are formed through the linkage of repeating units, called monomers, via covalent bonds. Organic polymers can be naturally occurring, derived from living organisms, or synthetic, synthesized through chemical processes.
They have a number of properties and applications, including flexibility, strength, thermal stability, biodegradability, and electrical conductivity. Examples of organic polymers include proteins, carbohydrates, synthetic plastics, and biopolymers.
In biological processes proteins function as enzymes, structural components, and signaling molecules. Collagen, for instance, forms the structural framework of connective tissues in animals, providing strength and elasticity to skin, tendons, and bones. Keratin, another protein polymer, constitutes the structural basis of hair, nails, and feathers, imparting strength and resilience to these tissues.
Carbohydrates serve as energy sources and structural materials in living organisms. Cellulose, a polysaccharide found in plant cell walls, provides rigidity and support to plant cells. Starch, another carbohydrate polymer, serves as a storage form of glucose in plants, providing a readily available energy source for metabolism. Other than in biological systems, carbohydrates are commonly used in industrial applications like food production, textiles, and pharmaceuticals.
Synthetic organic polymers, such as polyethylene, polyvinyl chloride (PVC), and polystyrene, are extensively utilized in modern industries due to their customizable properties and ease of processing. Polyethylene, for example, is one of the most produced plastics globally. PVC finds applications in construction, healthcare, and packaging industries whereas Polystyrene is used in packaging materials, disposable utensils, and insulation foams.
The synthesis of organic polymers involves polymerization reactions, wherein monomers are chemically bonded together to form long chains or networks. Polymerization processes can be initiated by various methods, including heat, light, or chemical initiators, and may occur via addition or condensation reactions.
Inorganic Polymers
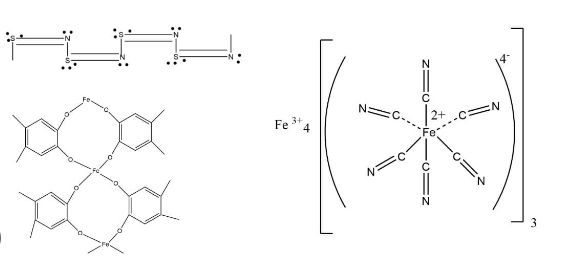
An inorganic polymer is a large molecule composed of elements other than carbon, such as silicon, oxygen, metals, and other non-carbon-based compounds. These polymers are formed through the linkage of repeating units, similar to organic polymers, but they do not contain carbon as the primary structural element.
Inorganic polymers can be naturally occurring, found in minerals and rocks, or synthetic, produced through chemical processes. Examples of inorganic polymers include silicones, silicates, certain ceramics, and metal oxides. These materials are used in industries like aerospace, electronics, construction, and automotive manufacturing, among others.
Silicones are one of the most well-known examples of inorganic polymers because of their thermal stability, and resistance to extreme temperatures and environmental conditions. These silicon-based polymers are synthesized through the polymerization of siloxane monomers, which consist of alternating silicon and oxygen atoms.
Silicates are minerals and materials composed of silicon, oxygen, and other elements. Clay minerals, for instance, are naturally occurring silicate polymers that have been used for millennia in ceramics, pottery, and construction materials due to their abundance, plasticity, and thermal stability.
Similarly, glass, a non-crystalline amorphous solid, is composed of silicon dioxide (silica) and other metal oxides, forming a network structure that imparts transparency, strength, and chemical inertness to the material.
Inorganic polymers also include certain ceramics, such as alumina (aluminum oxide) and zirconia (zirconium dioxide), which exhibit exceptional mechanical, thermal, and electrical properties. Unlike organic polymers, which are often biodegradable, inorganic polymers tend to be more resistant to degradation and environmental degradation.
Organic vs Inorganic Polymers in Tabular Form
| Basis | Organic Polymers | Inorganic Polymers |
|---|---|---|
| Chemical Composition | Composed primarily of carbon, hydrogen, oxygen, and nitrogen | Composed of elements such as silicon, oxygen, and metals |
| Source | Derived from living organisms or synthesized from natural sources | Can be naturally occurring (minerals) or synthetic |
| Examples | Proteins (e.g., collagen, keratin), carbohydrates (e.g., cellulose, starch), synthetic polymers (e.g., polyethylene, PVC) | Silicates (e.g., glass), silicones, certain ceramics |
| Bonding | Typically held together by covalent bonds | Bonding can be covalent, ionic, or metallic |
| Flexibility | Generally flexible and can exhibit elasticity | May be rigid or flexible depending on the structure |
| Biodegradability | Many organic polymers are biodegradable | Inorganic polymers are often non-biodegradable |
| Thermal Stability | Organic polymers generally have lower thermal stability | Inorganic polymers often have higher thermal stability |
| Electrical Conductivity | Organic polymers are typically poor conductors of electricity | Inorganic polymers can exhibit both conductivity and insulation properties |
| Solubility | Organic polymers can be soluble in certain solvents | Inorganic polymers are generally insoluble in solvents |
| Environmental Impact | Biodegradable organic polymers can have lower environmental impact | Inorganic polymers may persist in the environment for long periods |
| Applications | Commonly used in industries like healthcare, packaging, textiles, and construction | Utilized in industries such as aerospace, electronics, automotive, and construction |
| Synthesis | Can be synthesized from renewable resources or fossil fuels | Synthesized through various chemical processes, including polymerization of monomers |
Summary
Also Read: Difference Between Organic And Inorganic Chemistry
