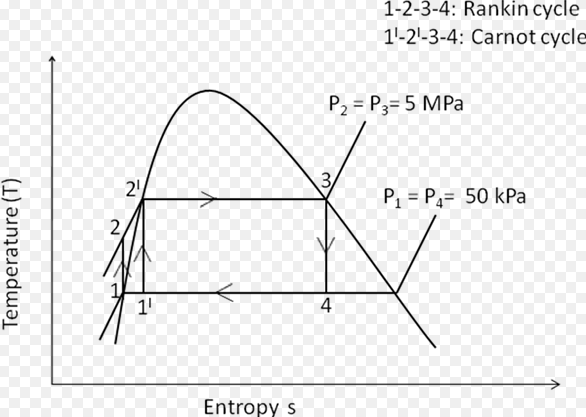
Difference Between Carnot Cycle And Rankine (With Diagram)
What is Carnot Cycle? The Carnot cycle was first proposed by a French engineer in 1824 and was expanded upon by others in 1830s and 1840s. It is an ideal cycle in which, the working medium receives the heat energy from the high temperatures and rejects the heat at the lower temperature. This cycle laid … Read more