Seaborgium (Sg) is a synthetic chemical element with the atomic number 106. It was first synthesized in 1974 by a team of scientists led by Albert Ghiorso at the Lawrence Berkeley National Laboratory in California, USA. Seaborgium was named in honor of Glenn T. Seaborg, an American chemist who was one of the principal discoverers of several transuranium elements, including plutonium and americium, and who also served as chairman of the United States Atomic Energy Commission.
In the periodic table of the elements, it is a d-block transactinide element. It is a member of the 7th period and belongs to the group 6 elements as the fourth member of the 6d series of transition metals. Chemistry experiments have confirmed that seaborgium behaves as the heavier homologue totungsten in group 6. The chemical properties of seaborgium are characterized only partly, but they compare well with the chemistry of the other group 6 elements.
In 1974, a few atoms of seaborgium were produced in laboratories in the Soviet Union and in the United States. The priority of the discovery and therefore the naming of the element was disputed between Soviet and American scientists, and it was not until 1997 that the International Union of Pure and Applied Chemistry (IUPAC) established seaborgium as the official name for the element. It is one of only two elements named after a living person at the time of naming, the other being oganesson, element 118
Seaborgium is a highly radioactive element and is produced by bombarding lighter elements with heavy ions in a particle accelerator. The most stable known isotope, 269Sg, has a half-life of approximately 14 minutes. Due to its high radioactivity and short half-life, seaborgium has no practical applications and is primarily of scientific interest for studying nuclear physics and the properties of superheavy elements.
Electron Configuration
The electron configuration of an element describes the distribution of its electrons among the various atomic orbitals. Seaborgium (Sg) is a synthetic element with the atomic number 106, which means it has 106 electrons.
Given its position in the periodic table, Seaborgium belongs to the d-block transition metals. Its electron configuration can be represented as follows:
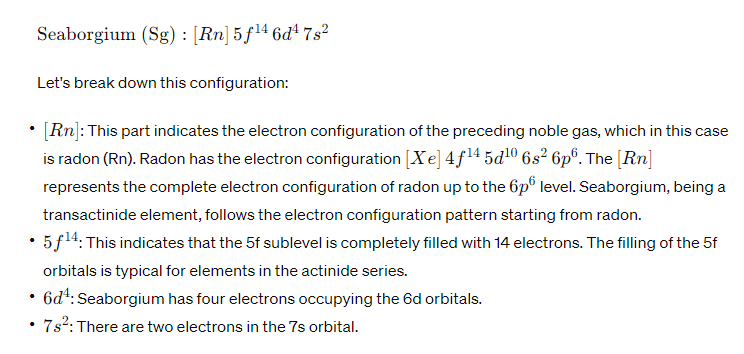
This electron configuration follows the Aufbau principle, Hund’s rule, and the Pauli exclusion principle, which govern the filling of atomic orbitals with electrons. Seaborgium, being a synthetic and highly unstable element, is of scientific interest, and its chemical and physical properties are still being studied.
Properties of Seaborgium
Please note that some of these values are estimated or predicted due to the difficulty in studying and observing Seaborgium due to its extremely short half-life and the challenges in synthesizing and isolating it.
| Atomic Number | 106 |
| Symbol | Sg |
| Atomic Mass | Approximately 269 u |
| Electron Configuration | [Rn] 5f^14 6d^4 7s^2 |
| Group | Transition metal |
| Period | 7 |
| Block | d-block |
| Element Category | Transition Metal |
| Density | Estimated around 35 to 40 g/cm^3 |
| Melting Point | Unknown (estimated to be around 1200°C) |
| Boiling Point | Unknown (estimated to be around 2500°C) |
| State at Room Temp. | Presumed to be solid |
| Oxidation States | +6, +5, +4 |
| Electronegativity | Estimated around 1.3 (Pauling scale) |
| Atomic Radius | Estimated around 130 pm |
| Covalent Radius | Estimated around 143 pm |
| Crystal Structure | Unknown |
| Discovery | Synthesized in 1974 at Lawrence Berkeley National Laboratory |
| Named After | Glenn T. Seaborg, an American chemist |
| Main Isotopes | Sg-266, Sg-267, Sg-268, Sg-269 |
| Most Stable Isotope | Sg-269, with a half-life of around 14 minutes |
Known Isotopes of Seaborgium (Sg)
Note: The half-lives provided are approximate values. Some of these isotopes might have had their properties updated or new isotopes discovered, its always important to check more recent sources for the latest information.
| Isotope | Symbol | Atomic Number (Z) | Number of Neutrons (N) | Mass Number (A) | Half-life | Decay Mode |
|---|---|---|---|---|---|---|
| Seaborgium-258 | Sg-258 | 106 | 152 | 258 | ~4.2 milliseconds | Unknown |
| Seaborgium-259 | Sg-259 | 106 | 153 | 259 | ~0.82 milliseconds | Unknown |
| Seaborgium-260 | Sg-260 | 106 | 154 | 260 | ~5.5 milliseconds | Unknown |
| Seaborgium-261 | Sg-261 | 106 | 155 | 261 | ~0.8 milliseconds | Unknown |
| Seaborgium-262 | Sg-262 | 106 | 156 | 262 | ~0.5 milliseconds | Unknown |
| Seaborgium-263 | Sg-263 | 106 | 157 | 263 | ~1.1 milliseconds | Unknown |