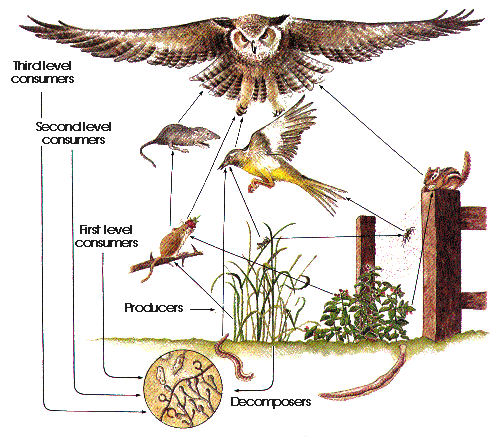
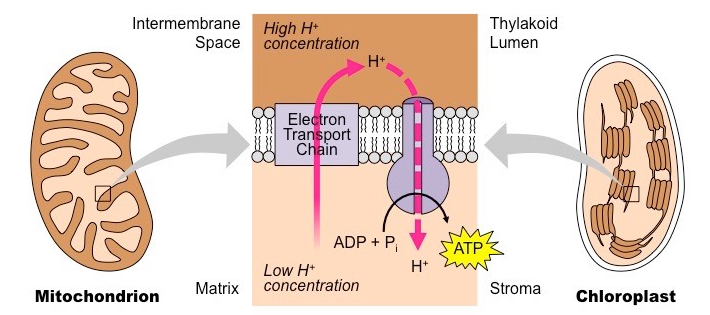
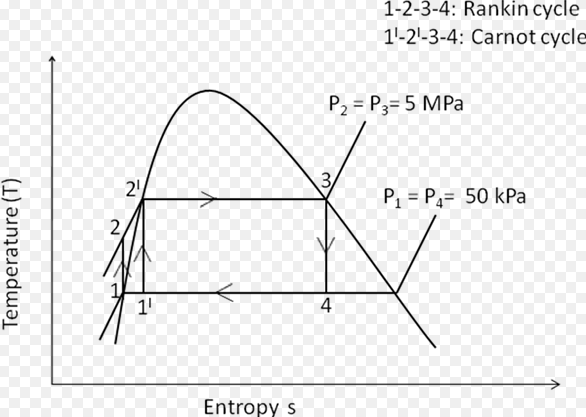
Difference Between Mold And Yeast (With Pictures)
Learn the primary differences between Mold and Yeast. The basis of comparison include: Definition reproduction, appearance and texture, examples, growth conditions, number of species, habitat, virulence, conversion of food substances, uses, cell structure, hyphae and health problems. What is Mold? Mold refers to a type of multicellular fungus that grows in the form of multicellular … Read more