Haemoglobin
Hemoglobin is the protein molecule in red blood cells that carries oxygen from the lungs to the body’s tissues and returns carbon dioxide from the tissues back to the lungs.
Hemoglobin is made up of four protein molecules (globulin chains) that are connected together. The normal adult hemoglobin (abbreviated Hgb or Hb) molecule contains two alpha-globulin chains and two beta-globulin chains. In fetuses and infants, beta chains are not common and the hemoglobin molecule is made up of two alpha chains and two gamma chains. As the infant grows, the gamma chains are gradually replaced by beta chains, forming the adult hemoglobin structure.
Each globulin chain contains an important iron-containing porphyrin compound termed heme. Embedded within the heme compound is an iron atom that is vital in transporting oxygen and carbon dioxide in our blood. The iron contained in hemoglobin is also responsible for the red color of blood.
Hemoglobin also plays an important role in maintaining the shape of the red blood cells. In their natural shape, red blood cells are round with narrow centers resembling a donut without a hole in the middle. Abnormal hemoglobin structure can, therefore, disrupt the shape of red blood cells and impede their function and flow through blood vessels.
Haemoglobin levels vary from person to person. Men usually have higher levels than women. A haemoglobin “cut-off” level is set for blood donation to ensure that your haemoglobin will not drop below normal after you have donated blood. Normal ranges for haemoglobin differ between ethnic populations, and males and females, and are also affected by age, especially in women. Individuals with haemoglobin levels below the normal range are, by definition, anaemic. There are many causes of anaemia and anaemia due to iron deficiency is common.
Facts About Hemoglobin
- Hemoglobin is an iron and oxygen-binding protein in blood, specifically red blood cell.
- Hemoglobin is abbreviated as Hb.
- Hemoglobin has a molecular weight of 64 kDa.
- Hemoglobin has 4 polypeptide chains: alpha, Beta, Delta and Gamma or epsilon (Depending on the type of hemoglobin).
- Systematically present all over the body.
- Hemoglobin occurs as a tetrameric protein and binds molecular oxygen on Red blood cells thereby ensuring that they are distributed throughout the body.
- Types of hemoglobin include Hemoglobin A, Hemoglobin A2 and Haemoglobin F.
- Takes oxygen from the lungs and transport to the rest of the body.
- Oxygen binding affected by PH and CO2.
- Haemoglobin exhibits a sigmoid curve in terms of oxygen dissociation (oxygen binding to hemoglobin at neutral PH).
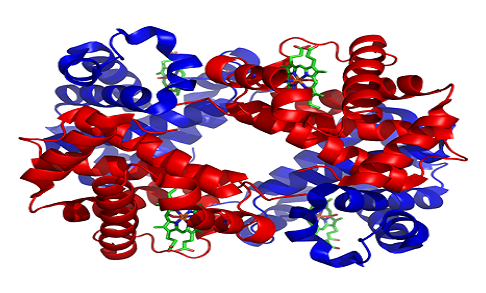
- Exhibits quaternary structure which is a characteristic of many multi-subunit globular proteins.
- Haemoglobin has a low affinity to bind with oxygen.
- In human, hemoglobin is found throughout the bloodstream.
Also Read: Difference Between White And Red Blood Cells
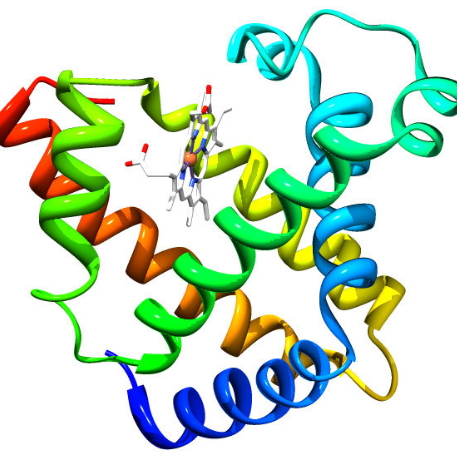
Myoglobin
Myoglobin is a small, monomeric protein which serves as an intracellular oxygen storage site. It is found in abundance in the skeletal muscle of vertebrates, and is responsible for the characteristic red color of muscle tissue. It traps oxygen within muscle cells, allowing the cells to produce the energy required for muscles to contract.
High concentrations of myoglobin in muscle cells allow organisms to hold their breath for a longer period of time. Diving mammals such as whales and seals have muscles with particularly high abundance of myoglobin.
When heart or skeletal muscle is injured, myoglobin is released into the blood. Elevated levels can be measured within a few hours following an injury.
Myoglobin is filtered from the blood by the kidneys and is released into the urine. Large quantities of myoglobin are toxic to the kidneys. If significant amounts of myoglobin are released into the bloodstream, which can happen after severe trauma or muscle injuries, the excess myoglobin may cause damage to the kidneys and eventually result in kidney failure. Measurement of myoglobin in urine helps to detect this condition.
There is a close chemical similarity between myoglobin and hemoglobin, the oxygen-binding protein of red blood cells. Both proteins contain a molecular constituent called heme, which enables them to combine reversibly with oxygen. The heme group, which contains iron, imparts a red-brown colour to the proteins. The bond between oxygen and hemoglobin is more complex than that between oxygen and myoglobin and accounts for the dual ability hemoglobin has to transport oxygen as well as to store it.
In contact with venous blood, oxygen combines more readily with myoglobin than it does with hemoglobin, favouring the transfer of oxygen from blood to muscle cells. Thus, the oxygen that the working muscle requires for the energy-producing biochemical reactions is provided.
Facts About Myoglobin
- Myoglobin is an iron and oxygen-binding protein found in the muscle tissue of vertebrates in general and in almost all mammals.
- Myoglobin is abbreviated as mb
- Myoglobin has a molecular weight of 16.7 kDa
- Myoglobin has a single polypeptide chains.
- Only present in heart and muscles.
- Myoglobin occurs as a monomeric protein and binds molecular oxygen and distributes it to the muscles.
- A single type of myoglobin is found in all cells.
- Stores oxygen in the muscle cells and releases it when it is needed.
- Oxygen binding in myoglobin is not affected by CO2 and PH.
- Myoglobin exhibits a hyperbolic curve in terms of oxygen dissociation.
- Exhibits tertiary structure.
- Has a high affinity to bind with oxygen, which does not depend on oxygen concentration.
- In humans, myoglobin is found in the bloodstream after muscle injury.
Also Read: Myoglobin Vs Haemoglobin Oxygen Dissociation Curve
Differences Between Hemoglobin And Myoglobin In Tabular Form
| BASIS OF COMPARISON | HEMOGLOBIN | MYOGLOBIN |
| Definition | Hemoglobin is a red protein which is responsible for transporting oxygen in the blood of vertebrates. | Myoglobin is a red protein with haem which carries and stores oxygen in muscle cells. |
| Molecular weight | Molecular weight is 64 kDa | Molecular weight is 16.7 kDa |
| Number of Chains | Haemoglobin has 4 polypeptide chains: alpha, Beta, Delta and Gamma or epsilon (Depending on the type of haemoglobin). | Myoglobin has a single polypeptide chains. |
| Location | Systematically present all over the body. | Only present in heart and muscles. |
| Also known as | Hb | Mb |
| Occurrence | Haemoglobin occurs as a tetrameric protein. | Myoglobin occurs as a monomeric protein. |
| Types | Types of haemoglobin include Haemoglobin A, Hemoglobin A2 and Haemoglobin F. | A single type of myoglobin is found in all cells. |
| Roles | Takes oxygen from the lungs and transport to the rest of the body. | Stores oxygen in the muscle cells and releases it when it is needed. |
| Oxygen Binding | Oxygen binding affected by PH and CO2. | Oxygen binding not affected by CO2 and PH. |
| Curves | Haemoglobin exhibits a sigmoid curve in terms of oxygen dissociation. | Myoglobin exhibits a hyperbolic curve in terms of oxygen dissociation. |
| Level of structure | Exhibits quaternary structure. | Exhibits tertiary structure. |
| Binding Affinity | Haemoglobin has a low affinity to bind with oxygen. | Has a high affinity to bind with oxygen, which does not depend on oxygen concentration. |
Also Read: Difference Between Lymph And Blood
Similarities Between Hemoglobin And Myoglobin
- Both myoglobin and hemoglobin give red color to the blood and muscles.
- Both contain oxygen-binding haem
- Both are globular proteins.