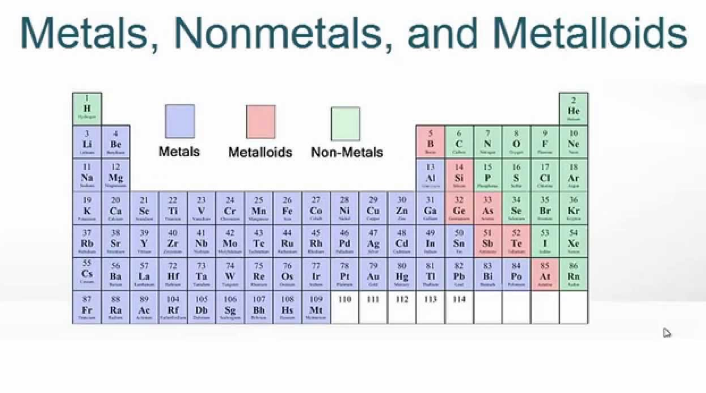
The periodic table, also known as the periodic table of the elements is a foundation of chemistry as it organizes all known chemical elements based on their atomic properties. Among the large number of elements, three categories stand out: metals, nonmetals and metalloids.
Let us learn the difference between metals, nonmetals and Metalloids. The basis of comparison include: description, appearance, malleability and ductility, position on the periodic table, electronegativity , conductivity of heat and electricity, boiling and melting points.
What are Metals?
A metal in chemistry can be defined as an element that readily forms positive ions and has metallic bonds. Metals make up the majority of the periodic table.
They are found on the left side and the middle of the periodic table. Metals can be recycled repeatedly without altering their properties, other key characteristics of metals include:
- Luster: Metals have a shiny, reflective surface due to their ability to reflect light.
- Conductivity: They are good conductors of heat and electricity. This property makes them essential in electrical wires and circuits.
- Malleability and Ductility: Metals can be easily hammered into thin sheets (malleability) and drawn into wires (ductility) without breaking.
- High Density and Melting Point: Metals are generally dense and have high melting points, making them suitable for structural applications.
- Chemical Reactivity: Metals tend to lose electrons easily and form positive ions in chemical reactions, leading to a characteristic tendency to react with nonmetals to form ionic compounds.
- Example Elements: Some common metals include iron, copper, aluminum, gold, and silver.
While metals share these common characteristics, there is a considerable variation in their properties within the group. For example, some metals are very reactive and readily corrode (like alkali metals), while others are highly resistant to corrosion (such as noble metals like gold and platinum).
What are Non-Metals?
Nonmetals are a group of elements that occupy the upper right-hand side of the periodic table. Unlike metals, nonmetals lack several of the characteristic properties commonly associated with metals.
Nonmetals have high ionization energies and have ability to gain electrons easily. Other Key characteristics of nonmetals include:
Some of the properties and characteristics that define nonmetals include:
- Lack of Luster: They generally have a dull or non-reflective appearance. They do not exhibit the shiny, metallic luster as seen in metals.
- Poor Conductivity: They are poor conductors of heat and electricity due to their higher resistance to electron flow.
- Brittle and Non-malleable: Most nonmetals are brittle and cannot be easily shaped into thin sheets or drawn into wires without breaking.
- Lower Density and Melting Point: They usually have lower densities and melting points compared to metals.
- Electron Gainers: In chemical reactions, they tend to gain electrons rather than lose them. This results in the formation of negatively charged ions (anions) when they react with metals to form ionic compounds.
- Variability in Chemical Reactivity: Some nonmetals, like fluorine and oxygen, are highly reactive and can readily form compounds with other elements. Noble gases (a group of nonmetals) are generally inert and do not readily react with other elements.
- Covalent Bonds: Nonmetals are crucial in forming covalent bonds, where atoms share electrons to achieve a stable electron configuration. Covalent bonds usually occur in nonmetal-containing molecules and compounds.
Also Read: Difference Between Ferrous And Nonferrous Metals
What are Metalloids?
A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. They are found in a staircase-like pattern along the diagonal border between metals and nonmetals in the periodic table. In other words, they are located to the right of metals and to the left of nonmetals in the periodic table. They are solids under standard conditions and can easily form alloys with Metals.
Some the elements that are generally considered metalloids include: boron, arsenic, antimony, silicon, germanium, selenium, polonium and tellurium.
Some of the key characteristics and properties of metalloids:
- Semi-Conductivity: They are not as efficient conductors as metals but can conduct better than typical nonmetals. This property makes metalloids crucial in electronic devices as semiconductors. Silicon, in particular, is one of the most widely used semiconductors in the electronics industry.
- Varied Physical Properties: They a mix of physical properties. For example, boron is a relatively hard, lustrous solid, while carbon (specifically in the form of graphite) is a black, opaque, soft material.
- Amphoteric Nature: Some metalloids, like aluminum and zinc, display amphoteric behavior, meaning they can act as both acids and bases in chemical reactions.
- Allotropes: Several metalloids can exist in different allotropes, which are different structural forms of the same element. For instance, carbon has various allotropes, such as diamond, graphite, and fullerenes (like buckyballs and nanotubes).
- Chemical Reactivity: They can display both metallic and nonmetallic reactivity. For example, boron acts as a nonmetal in its elemental form but exhibits some metallic behavior when combined with other elements.
- Electronegativity: They have intermediate electronegativity values, which means they can form both covalent and ionic compounds with other elements.
Also Read: Difference Between Cations And Anions
Metals vs Nonmetals vs Metalloids In Tabular Form
| METALS | NONMETALS | METALLOIDS |
| A metal in chemistry can be defined as an element that readily forms positive ions and has metallic bonds. | A non-metal is an element that does not possess the properties of a metal, that is, can’t be made into wire; neither can it conduct heat nor electricity. | A metalloid is an element that has properties that are intermediate between those of metals and nonmetals. |
| Metals are shiny in appearance. | Have a dull or non-reflective appearance. | May have a metallic luster but less pronounced than metals. |
| Metals are good conductors of both heat and electricity. | Nonmetals are bad conductors of heat and electricity. | Intermediate conductors, exhibiting semiconducting properties. |
| Metals have a very low electronegativity. | Nonmetals have a high electronegativity. | Metalloids have an intermediate value of electronegativity. |
| Malleable (can be hammered into thin sheets) and ductile (can be drawn into wires). | Brittle and non-malleable, they shatter when hammered. | Some metalloids may exhibit limited malleability and ductility. |
| Metals are found in S, P, D and F blocks of the periodic table. | Nonmetals are found in S and P blocks of the periodic table. | Metalloids are found in P block. |
| Predominantly found on the left and middle portions of the periodic table. | Primarily found on the right side of the periodic table. | Located in a diagonal band between metals and nonmetals. |
| Generally, have high densities and melting points. | Usually have lower densities and melting points compared to metals. | Their densities and melting points vary within the group. |
| Tend to lose electrons and form positive ions (cations) in chemical reactions. | Tend to gain electrons and form negative ions (anions) in chemical reactions. | Can exhibit both electron donor and acceptor behaviors, depending on the specific element. |
| Tend to form ionic bonds with nonmetals by transferring electrons. | Participate in ionic bonding by accepting electrons from metals. | Can participate in both ionic and covalent bonding, depending on the context. |
| Most metals are solid at room temperature, with mercury being a notable exception (liquid metal). | Can exist in various states at room temperature, with some gases (oxygen) and some solids (sulfur). | Tend to be solids at room temperature, but silicon and germanium are also used in semiconductor |
| Typically form positive oxidation states (cations) in chemical compounds. | Form negative oxidation states (anions) or share electrons in covalent bonds. | Can exhibit various oxidation states depending on the specific element and chemical environment. |
| Examples: Iron, copper, aluminum, gold, silver. | Example: Carbon, nitrogen, oxygen, sulfur, hydrogen. | Example: Boron, silicon, germanium, arsenic, antimony, tellurium. |