Among the many classifications of sugar, “reducing sugar” and “non-reducing sugar” stand out as two intriguing categories that often confuse both culinary enthusiasts and scientific minds alike.
What Are Reducing Sugars?
Reducing sugar is any carbohydrate which is capable of being oxidized and causes the reduction of other substances without having to be hydrolyzed first. Reducing sugars are carbohydrates that can act as reducing agents due to the presence of free aldehyde groups or free ketone groups.
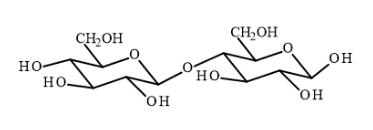
Examples of reducing sugars:
- Maltose
- Lactose
- Melibiose
- Cellobiose
- Gentiobiose
- Fructose
- Mannose
- Galactose
- Glucose
What You Need To Know About Reducing Sugars
- Reducing sugar is any carbohydrate which is capable of being oxidized and causes the reduction of other substances without having to be hydrolyzed first.
- Reducing sugars are carbohydrates that can act as reducing agents due to the presence of free aldehyde groups or free ketone groups.
- Reducing sugars have a sweet taste.
- Most of the reducing sugars are Monosaccharides.
- Reducing sugars give a dark red color (brick color) when they react with Benedict solution.
- Reducing sugar has a free aldehyde (-CHO) or ketonic (-CO) group.
- Reducing sugars give a positive reaction towards the Fehling’s test.
- Reducing sugars have the capacity to reduce cupric ions of Benedict’s or Fehling solution to cuprous ions.
- Presence or absence of reducing sugars can be identified by carrying out different tests.
- Generally, the molecular weight of reducing sugars is relatively low.
What Are Non-reducing Sugars?
Non-reducing sugars are any type of carbohydrate which are unable to be oxidized and do not reduce other substances. Non-reducing sugars are carbohydrates that cannot act as reducing agents due to the absence of free aldehyde groups or free ketone groups.
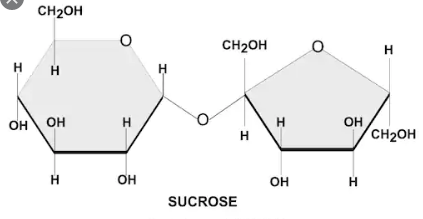
Examples of Non-reducing sugars include:
- Sucrose
- Trehalose
- Raffinose
- Stachyose
- Verbascose
What You Need To Know About Non-reducing Sugars
- Non-reducing sugars are any type of carbohydrate which are unable to be oxidized and do not reduce other substances.
- Non-reducing sugars are carbohydrates that cannot act as reducing agents due to the absence of free aldehyde groups or free ketone groups.
- Non-reducing sugars have a less sweet taste.
- Most of non-reducing sugars are polysaccharides while others are disaccharides.
- Non-reducing sugars do not give a red color when they react with Benedict’s solution, instead remains as green in color.
- Non-reducing does not have a free aldehyde or ketonic group.
- Non-reducing sugars give a negative reaction towards the Fehling’s test.
- Non-reducing sugar fail to reduce the cupric ions of Benedict’s solution to coprous ions.
- The presence or absence of non-reducing sugars cannot be identified by different tests.
- Generally, the molecular weight of reducing sugars is relatively high when compared to that of reducing sugars.
Also Read: Difference Between Ketose And Aldose
Difference Between Reducing Sugar And Non-reducing Sugar In Tabular Form
| BASIS OF COMPARISON | REDUCING SUGARS | NON-REDUCING SUGARS |
| Description | Reducing sugar is any carbohydrate which is capable of being oxidized and causes the reduction of other substances without having to be hydrolyzed first. | Non-reducing sugars are any type of carbohydrate which are unable to be oxidized and do not reduce other substances. |
| Role | Reducing sugars are carbohydrates that can act as reducing agents due to the presence of free aldehyde groups or free ketone groups. | Non-reducing sugars are carbohydrates that cannot act as reducing agents due to the absence of free aldehyde groups or free ketone groups. |
| Sweet Taste | Reducing sugars have a sweet taste. | Non-reducing sugars have a less sweet taste. |
| Sugar Classes | Most of the reducing sugars are Monosaccharides. | Most of non-reducing sugars are polysaccharides while others are disaccharides. |
| Reaction With Benedict Solution | Reducing sugars give a dark red color (brick color) when they react with Benedict solution. | Non-reducing sugars do not give a red color, instead remains as green in color. |
| Fehling’s Test | Reducing sugars give a positive reaction towards the Fehling’s test. | Non-reducing sugars give a negative reaction towards the Fehling’s test. |
| Presence Of Aldehyde or Ketonic Group | Reducing sugar has a free aldehyde (-CHO) or ketonic (-CO) group. | Non-reducing does not have a free aldehyde or ketonic group. |
| Ability To Reduce Cupric Ions | Reducing sugars have the capacity to reduce cupric ions of Benedict’s or Fehling solution to cuprous ions. | Non-reducing sugar fail to reduce the cupric ions of Benedict’s solution to coprous ions. |
| Tests | Presence or absence of reducing sugars can be identified by carrying out different tests. | The presence or absence of non-reducing sugars cannot be identified by different tests. |
| Molecular Weight | The molecular weight of reducing sugars is relatively low. | The molecular weight of reducing sugars is relatively high when compared to that of reducing sugars. |
Also Read: Difference Between Monosaccharide, Disaccharide And Polysaccharide
Key Difference
- Reducing sugars are sugars that have a free aldehyde or ketone group in their molecular structure, which can undergo a redox (oxidation-reduction) reaction. Common reducing sugars include glucose, fructose, and lactose. Non-reducing sugars are sugars that lack a free aldehyde or ketone group and, therefore, cannot undergo a redox reaction. Sucrose is a typical non-reducing sugar.
- When reducing sugars are exposed to Fehling’s solution or Benedict’s reagent (both contain copper ions), they can reduce the copper ions, resulting in a color change from blue to brick-red precipitate. Non-reducing sugars do not react with Fehling’s or Benedict’s reagent, so there is no color change observed in the solution.
- Reducing sugars sugars contain either an aldehyde (e.g., glucose) or a ketone (e.g., fructose) functional group, which allows them to act as reducing agents in various chemical reactions. Non-reducing sugars lack the necessary functional group (aldehyde or ketone) and are unable to act as reducing agents.
- Reducing sugars are capable of being hydrolyzed by specific enzymes or acidic conditions into their constituent monosaccharides. For example, maltose (a reducing disaccharide) can be hydrolyzed into two glucose molecules. Non-reducing sugars, like sucrose, are resistant to hydrolysis under normal conditions due to the absence of a free aldehyde or ketone group.
Comments are closed.