A carbohydrate is an organic compound consisting of carbon, hydrogen and oxygen. Carbohydrates are classified into monosaccharides, disaccharides, oligosaccharides and polysaccharides. The monosaccharides are the simplest forms of carbohydrates and are further classified based on the carbonyl group they contain. Accordingly, monosaccharides are the simplest form of carbohydrates and may be subcategorized as Aldoses or ketoses.
What Is Aldose?
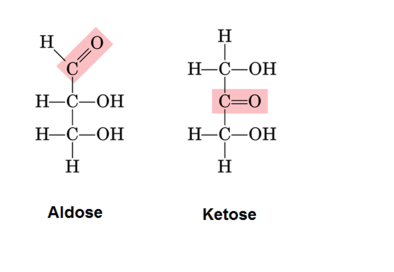
An Aldose is a monosaccharide (simple sugar) with carbon backbone chain with a carbonyl group on the endmost carbon atom, making it an aldehyde and hydroxyl groups connected to all other carbon atoms. In organic chemistry, an aldehyde functional group is defined by the presence of a carbon atom double bonded to an oxygen atom and single bonded to a hydrogen atom.
Majority of the Aldose molecules are cyclical in structure. Usually, when molecules have cyclical structures, they form a six-member ring structure referred to as hemiacetal ring because of the presence of carbon. In Seliwanoff’s test, (where the sample is heated with acid and resorcinol), Aldoses tend to respond at a moderate pace and deliver a slow light pink color.
The chemical names of the Aldose sugars depend on the number of carbon atoms they possess. The minimum number of carbons in a backbone needed to form a molecule that is still considered a carbohydrate is 3, and carbohydrates with three carbons are referred to as trioses. The only aldotriose is glyceraldehyde. The common categories of Aldoses are those with 6 carbons, aldohexoses.
Examples of Aldoses include:
- Glyceraldehyde
- Erythrose
- Ribose
- Glucose
- Galactose
What You Need To Know About Ketose Sugar
- Aldose is the monosaccharide that contains aldehyde group in its structure along with the carbon chain.
- Aldose structure has one carbon atom.
- The chemical formula of Aldose is written as Cn(H2O)n.
- Aldose is a pure sugar.
- Examples of Aldose are Glycolaldehyde, glyceraldehydes, erythrose, threose, glucose and galactose .
- In Seliwanoff’s test, (where the sample is heated with acid and resorcinol), Aldoses tend to respond at a moderate pace and deliver a slow light pink color.
- An Aldose may decompose into Ketose depending on the isomerization reaction.
- Aldoses are primarily found in plants. A good example is glucose.
- Majority of the Aldose molecules are cyclical in structure. Usually, when molecules have cyclical structures, they form a six-member ring structure referred to as hemiacetal ring because of the presence of carbon.
- The chemical names of the Aldose sugars depend on the number of carbon atoms they possess. The minimum number of carbons in a backbone needed to form a molecule that is still considered a carbohydrate is 3, and carbohydrates with three carbons are referred to as trioses. The only aldotriose is glyceraldehyde. The common category of Aldoses are those with 6 carbons, aldohexoses.
What Is Ketose?
Ketose is the monosaccharide that contains ketone group along with the carbon chain. All monosaccharide ketoses are reducing sugars. One way to chemically identify a ketose from an Aldose is through Seliwanoff’s test. In this test, Ketoses react with the crystalline compounds whose name is resorcinol to give a deep cheery-red color. To isomerize a ketose into an Aldose, Lobry-de Bruyn-van Ekenstein transformation is applied.
Ketoses may be further subcategorized based on the number of carbon in the main chain. For example, a three-carbon ketose is referred to as a triose, tetroses are four-carbon ketoses, pentoses are five-carbon ketoses, hexoses are six-carbon ketoses.
Examples of ketoses include:
- Trioses: dihydroxyacetone
- Tetroses: erythrulose
- Pentoses: ribulose, xylulose
- Hexoses: Fructose, psicose, sorbose, tagatose
- Heptoses: sedoheptulose
- Octoses: D-manno-octulose
- Nonoses: D-glycero-D-galacto-nonulose
What You Need To Know About Aldose Sugar
- Ketose is the monosaccharide that contains ketone group along with the carbon chain.
- Ketose structure has three carbon atoms.
- The chemical formula of ketose is written as RCOR. An R group is any molecule or atom that can bind to the carbonyl atom (CO), forming an aldehyde.
- Ketose is an impure sugar.
- Examples of ketone are Fructose, ribulose and xylulose, erythrulose, tagatose, sorbose, pentoses, hexoses, heptoses, octoses, nonoses, tetroses etc.
- In Seliwanoff’s test, Ketoses reacts with the crystalline compounds whose name is resorcinol to give a deep cheery-red color.
- Ketose can isomerizes into aldoses only if the carbonyl group is at the end of the chain.
- Ketoses can be found in processed foods. A good example is fructose.
- The carbon atom in the ketone group always gets the number 2. If Aldose forms a six-member ring, ketoses, like the fructose forms a five member ring referred to as hemiketal.
- The chemical names of the ketose sugars depend on the number of carbon atoms they possess. If there are five carbon atoms, it will be referred to as ketopentose and so on.
Also Read: Difference Between Reducing And Non-reducing Sugar
Difference Between Aldose And Ketose Sugar In Tabular Form
| BASIS OF COMPARISON | ALDOSE SUGAR | KETOSE SUGAR |
| Description | Aldose is the monosaccharide that contains aldehyde group in its structure along with the carbon chain. | Ketose is the monosaccharide that contains ketone group along with the carbon chain. |
| Number Of Carbon Atoms | Aldose structure has one carbon atom. | Ketose structure has three carbon atoms. |
| Chemical Formula | The chemical formula of Aldose is written as Cn(H2O)n. | The chemical formula of ketose is written as RCOR. |
| Chemical Purity | Aldose is a pure sugar. | Ketose is an impure sugar. |
| Examples | Examples of Aldose are Glycolaldehyde, glyceraldehydes, erythrose, threose, glucose and galactose . | Examples of ketone are Fructose, ribulose and xylulose, erythrulose, tagatose, sorbose, pentoses, hexoses, heptoses, octoses, nonoses, tetroses etc. |
| Seliwanoff’s Test | In Seliwanoff’s test, (where the sample is heated with acid and resorcinol), Aldoses tend to respond at a moderate pace and deliver a slow light pink color. | In Seliwanoff’s test, Ketoses reacts with the crystalline compounds whose name is resorcinol to give a deep cheery-red color. |
| Isomerization | An Aldose may decompose into Ketose depending on the isomerization reaction. | Ketose can isomerizes into aldoses only if the carbonyl group is at the end of the chain. |
| Source | Aldoses are primarily found in plants. A good example is glucose. | Ketoses can be found in processed foods. A good example is fructose. |
| Carbon Atom | Majority of the Aldose molecules are cyclical in structure. Usually, when molecules have cyclical structures, they form a six-member ring structure referred to as hemiacetal ring because of the presence of carbon. | The carbon atom in the ketone group always gets the number 2. If Aldose forms a six-member ring, ketoses, like the fructose forms a five member ring referred to as hemiketal. |
| Naming | The chemical names of the Aldose sugars depend on the number of carbon atoms they possess. The minimum number of carbons in a backbone needed to form a molecule that is still considered a carbohydrate is 3, and carbohydrates with three carbons are referred to as trioses. The only aldotriose is glyceraldehyde. The common category of Aldoses are those with 6 carbons, aldohexoses. | The chemical names of the ketose sugars depend on the number of carbon atoms they possess. If there are five carbon atoms, it will be referred to as ketopentose and so on. |
Also Read: Difference Between Monosaccharide, Disacharide And Polysaccharide
Comments are closed.