The triple point and critical point are both found on a phase diagram. They are terms used to explain temperatures and pressures at which two or more phases of substances can coexist with each other. The critical point is the condition at which the liquid and vapour phase of the same substance coexist. The triple point is the condition at which all three phases of matter can coexist with each other. In this article, find more about phase diagram, its application in real life and more about critical and triple points.
What is phase diagram?
A phase diagram is a representation of all the temperature and pressure combinations that create the different phases in a substance. Generally, the solid is left and top, liquid is in the middle and gas fills in the rest, until critical point is reached. The phase diagram has lines between the states indicating equilibrium points between two states of matter. The point where the lines converge is known as the triple point, which is a state of equilibrium where solid, liquid and gas exist together.
The following is an example of a phase diagram for a generic single-component system:
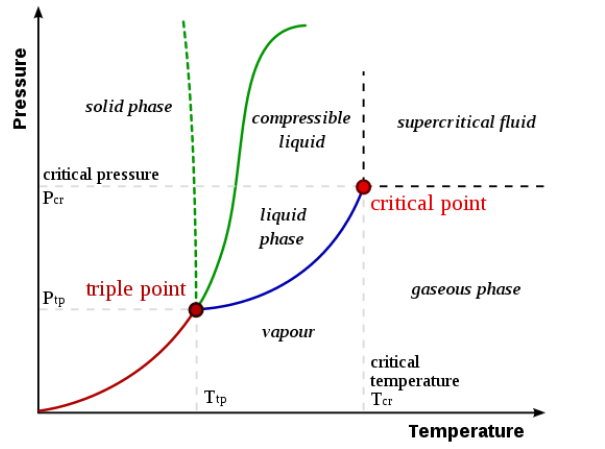
Phase diagrams plot pressure (typically in atmospheres) versus temperature (typically in degrees Celsius or Kelvin). The labels on the graph represent the stable states of a system in equilibrium. The lines represent the combinations of pressures and temperatures at which two phases can exist in equilibrium.
In other words, these lines define phase change points. The red line divides the solid and gas phases, represents sublimation (solid to gas) and deposition (gas to solid). The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid).
There are also two important points on the diagram, the triple point and the critical point. The triple point represents the combination of pressure and temperature that facilitates all phases of matter at equilibrium. The critical point terminates the liquid/gas phase line and relates to the critical pressure, the pressure above which a supercritical fluid forms.
With most substances, the temperature and pressure related to the triple point lie below standard temperature and pressure and the pressure for the critical point lies above standard pressure. Therefore at standard pressure as temperature increases, most substances change from solid to liquid to gas, and at standard temperature as pressure increases, most substances change from gas to liquid to solid.
What is triple point?
Triple point on a phase diagram represents the point where the three lines of equilibrium between states of matter converge. In other words, The triple point represents a temperature and pressure combination where all three states of matter exist in equilibrium. The triple point is a temperature and pressure combination. At this point, all three states of solid, liquid, and gas will exist simultaneously. Knowing this temperature and pressure combination helps with identifying the compounds in a substance.
What is critical point?
The critical point on a phase diagram represents the temperature and pressure combination in which the liquid and vapor form of the substance in question both become indistinguishable from each other. In other words, Critical point is the temperature and pressure combination where the gas form of a substance can no longer be condensed back to a liquid, which becomes a supercritical fluid. The critical point is generally labeled at the end of the equilibrium line between the liquid and gas phase on a phase diagram.
Critical Point vs Triple Point In Tabular Form
| Points of Comparison | Critical Point | Triple Point |
| Description | Critical point describes the coexistence of two phases of the same substance. | Triple point describes the coexistence of three phases of the same substance. |
| Phase Diagram | The point at which saturated liquid and saturated vapor lines meet on a phase diagram is the critical point. | The point at which fusion line and sublimation line and vaporization meet on a phase diagram is the triple point. |
| Temperature | For most of substances the critical temperature is usually higher than the standard temperature. | The temperature corresponding to the triple point is usually lower than the standard temperature. |
| Coexistence At Equilibrium | At the critical point, only liquid and gaseous phases can coexist in equilibrium. | At triple point, all solid, liquid and gaseous phases can coexist in equilibrium. |
| Representation | Critical point is represented by a point on p-v-T surface. | Triple point is represented by a point on p-T diagram but appears as a line on p-v-T surface. |
| Example | An example of critical point is the liquid-vapor critical point, the end point of the pressure-temperature curve that designates conditions under which a liquid and its vapor can coexist. | An example of triple point is the triple point of mercury, the triple point of mercury occurs at a temperature of -38.83440 degrees Celsius and a pressure of 0.2 mPa. |
| Values | The critical point of water is at 647K and 22.064 mPa. | The triple point of water is at 273.16 K and 0.6116557 mPa. |
The Key Takeaways
- Phase diagrams illustrate the variations between the states of matter of elements or compounds as they relate to pressure and temperatures.
- The triple point represents a temperature and pressure combination where all three states of matter exist in equilibrium.
- Critical point is the temperature and pressure combination where the gas form of a substance can no longer be condensed back to a liquid, which becomes a supercritical fluid.
- The critical point is the end point of a phase diagram curve whereas Triple point is the point at which all the boundary line meet with each other.
- The critical temperature is usually higher than the standard temperature.
- The temperature corresponding to the triple point is usually lower than the standard temperature.
- The triple point of water is at 273.16 K and 0.611657 MPa. The critical point of water is at 647 K and 22.064 MPa.
- With most substances, the temperature and pressure related to the triple point lie below standard temperature and pressure and the pressure for the critical point lies above standard pressure.