Hydrocarbons are compounds that are made up of atoms of carbon and hydrogen exclusively. The unique nature of the carbon ensures that it shares a strong covalent bond with hydrogen. Since carbon atoms will make long chains with relative ease, hydrocarbons can be very big molecules linking even hundreds of atoms. Examples of hydrocarbons include natural gas, coal, petrol, fossil fuel, cooking oil and even butter.
Hydrocarbons are broadly classified into two types, namely saturated and unsaturated. Saturated hydrocarbons have a single bonding between their atoms. Which means only one pair of electron is shared between any two atoms of the compound. Unsaturated hydrocarbons can have double or triple bonds.
What Are Alkanes?
Alkanes are organic compounds that consist entirely of single-bonded carbon and hydrogen atoms and lack any other functional groups. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree like structure in which all the carbon-carbon bonds are single. Alkanes have the general formula CnH2n+2 and can be subdivided into the following three groups: the linear straight-chain alkanes, branched alkanes and cycloalkanes.
Alkanes have the general formula CnH2n+2. For example, an alkane with 2(n) carbon atoms, will have 6 (2n+2) hydrogen atoms. Their adjacent atoms are connected with sigma bonds and form tetrahedral centers around the carbon atoms. As these bonds are all single bonds, there is free rotation around all connections. Each carbon atom has four bonds (either C-H or C-C) and each hydrogen atom is joined to a carbon atom (H-C bonds). A series of linked carbon atoms is known as the carbon skeleton or carbon backbone.
Examples of Alkanes include:
- Methane (CH4)
- Ethane (C2H6)
- Propane (C3H8)
- Butane (C4H10)
- Pentane (C5H12)
- Hexane (C6H14)
Structure Of Alkane
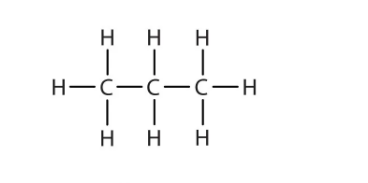
Properties Of Alkanes
- Alkanes are organic compounds that consist entirely of a single-bonded carbon and hydrogen atoms and lack any other functional groups.
- Alkanes have the general formula CnH2n+2
- They can be subdivided into three groups: the linear straight-chain alkanes, branched alkanes and cycloalkanes.
- Alkanes are typically colorless. Alkanes containing 5 carbons up to about 19 are colorless liquids whereas those with more than 20 carbon atoms are colorless, waxy solids.
- The alkanes can exist as gases, liquids or solids at standard ambient temperature and pressure.
- Due to very small difference of electronegativity between carbon and hydrogen and covalent nature of C-C bond or C-H bond, alkanes are generally non-polar molecules.
- Alkanes are generally insoluble in polar solvents like water but readily soluble in nonpolar solvents.
- The melting and boiling points of the shorter chain alkanes is low, but the melting and boiling points alkanes increase as the number of carbon atoms in the carbon chain increases.
- Alkanes are relatively unreactive. They do not react with strong acids, bases, oxidizing agents or reducing agents.
- Alkanes rapidly react with oxygen releasing energy, which makes alkanes useful as fuels.
- Alkanes react with halogens such as chlorine gas and bromine water in the presence of ultraviolet light.
- Alkanes do not conduct electricity in any way nor are they substantially polarized by an electric field.
- The density of the alkanes usually increases with the increase in number of carbon atoms but always remains less than that of water.
- Alkanes will react with steam in the presence of nickel to give hydrogen.
What Are Alkenes?
Alkenes are an unsaturated form of hydrocarbons that are formed by double bonding between the carbon atoms. There is at least one such double bond in their structure (C=C). Alkenes are generally prepared through elimination reactions in which two atoms on adjacent carbon atoms are removed, resulting in the formation of a double bond. Preparations include the dehydration of alcohols, the dehydrogenation of alkyl halides and the dehalogenation of alkanes.
Many of the physical properties of alkenes and alkanes are similar: they are colorless, Nonpolar and combustible. Alkenes exist naturally in all three states. The first three alkenes are gases and the next fourteen are liquids. Alkenes higher than these are all solids. The melting point of the solids also increases with increase in molecular mass.
Alkenes generally have stronger smells than their corresponding alkanes. Ethylene has a sweet and musty odor. Alkenes are relatively stable compounds, but are more reactive than alkanes, either because of the reactivity of the carbon-carbon double bond or the presence of allylic CH centers.
Examples Of Alkenes Include:
- Ethene (C2H4)
- Propene (C3H6)
- Butene (C4H8)
- Pentene (C5H10)
- Hexane (C6H12)
Structure Of Alkene
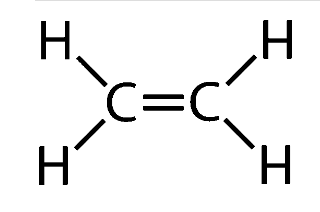
Properties Of Alkenes
- Alkenes are an unsaturated form of hydrocarbons that are formed by double bonding between the carbon atoms. There is at least one such double bond in their structure (C=C).
- The general formula for alkenes in the case of a non-cyclic compound is CnH2n.
- Alkenes are more reactive than alkanes due to presence of a double bond.
- Alkenes exist naturally in all three states. The first three alkenes are gases and the next fourteen are liquids. Alkenes higher than these are all solids.
- Alkenes are insoluble in water but readily soluble in organic solvents such as acetone or benzene.
- The boiling points of alkenes depend on their molecular structure. The bigger their molecular chain the higher the boiling points.
- The polarity of alkenes will depend on their functional group.
- Alkenes generally have stronger smells than their corresponding alkanes.
- Alkenes can be produced or synthesized by elimination reactions involving alkanols or haloalkanes.
- Alkenes will undergo complete combustion in excess oxygen and incomplete combustion if the supply of oxygen is limited.
- Alkenes undergo addition reactions with hydrogen, halogens, hydrogen halides and water.
Also Read: Difference Between Ionic And Covalent Bonds
What Are Alkynes?
An alkyne is an unsaturated hydrocarbon containing at least one carbon-carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional group form a homologous series with the general chemical formula CnH2n-2. Since it is also an unsaturated hydrocarbon, some of its properties will be similar to alkenes.
The first three alkynes are gases, and the next eight are liquids. All alkynes higher than these eleven are solids. The boiling and melting point of alkynes increase as their molecular structure grows bigger. The boiling point increases with increase in their molecular mass. Also, the boiling point of alkynes is slightly higher than those of their corresponding alkenes, due to the one extra bond at the carbon site.
Alkynes are generally nonpolar molecules with little solubility in polar solvents, such as water. They are readily soluble in nonpolar solvents such as ether and acetone. Also the acidity of these triple bonded compounds is greater than the acidity of alkanes and alkenes. Examples of alkynes include:
- Acetylene (ethyne)-C2H2
- Propyne (C3H4)
- Butyne (C4H6)
- Pentyne (C5H8)
- Hexyne (C6H10)
Structure Of Alkyne
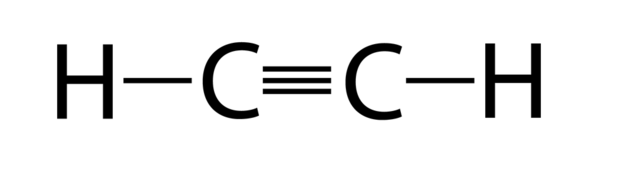
Properties Of Alkynes
- Alkynes are unsaturated carbon that shares a triple bond at the carbon site.
- The general formula for alkynes in the case of a non-cyclic compound is CnH2n-2.
- The first three alkynes are gases while those containing five to thirteen carbon atoms are liquids and higher alkynes are solids.
- All alkynes are odorless and colorless with the exception of ethylene which has a slight distinctive color.
- The melting and boiling points of alkynes are quite low and increase regularly with increase in molecular mass. Alkynes are less volatile than alkanes and alkenes.
- Alkynes are insoluble in water but are soluble in organic solvents such as benzene, hexane, ether, carbon tetrachloride etc.
- All alkynes are lighter than water. Their densities increase regularly with increase in molecular mass.
- Boiling points of alkynes are slightly higher than those of their corresponding alkenes due to one extra bond at the carbon site.
- Alkynes are slightly electronegative in nature. The triply bonded carbon atoms in alkynes are sp hybridized.
- Alkynes react with strong bases such as sodium and sodamide to form sodium alkynide with liberation of di-hydrogen gas. Such a reaction will not happen in alkanes and alkenes.
- Under standard conditions (temperature and pressure) alkynes will undergo hydration reaction quite easily.
- Alkynes are more acidic than alkanes and alkenes.
- When an alkyne is reacted with bromine, the reddish brown color of bromine vanishes.
- Alkynes also undergo polymerization reactions. For example, linear polymerization of ethyne under suitable conditions to produce polyacetylene (polyethyne) having formula –(C2H2)n-.
Also Read: Difference Between Aliphatic And Aromatic Compound
Difference Between Alkane, Alkene And Alkyne In Tabular Form
| BASIS OF COMPARISON | ALKANES | ALKENES | ALKYNES |
| Description | Alkanes are organic compounds that consist entirely of a single-bonded carbon and hydrogen atoms and lack any other functional groups. | Alkynes are unsaturated carbon that shares a double bond at the carbon site. | Alkynes are unsaturated carbon that shares a triple bond at the carbon site. |
| Alternative Name | Also referred to as Paraffin. | Also referred to as olefin. | Also referred to as acetylene. |
| Saturation | Saturated hydrocarbons. | Unsaturated hydrocarbons. | Unsaturated hydrocarbons. |
| General Formulae | CnH2n+2 | CnH2n. | CnH2n-2. |
| Carbon Bonds | Single (-C) | Double (C=C) | Triple (-C=) |
| Stability | Most stable hydrocarbon. | Less stable hydrocarbon than alkane. | Less stable hydrocarbon than Alkene. |
| State At Room Temperature & Pressure | Solid, liquid and gas | Solid, liquid and gas | Solid, liquid and gas |
| Reaction | Undergoes substitution reaction. | Undergoes addition reaction. | Undergoes addition reaction. |
| Boiling Point | Boiling point is lower than that of alkenes. | Boiling point is higher than that of alkane. | Boiling point is higher than that of alkenes and alkanes. |
| Reaction With KMnO4 | Addition of few drops of KMnO4 to ethane, no color change is observed. | Addition of few drops of KMnO4 to ethene, the purple color changes to colorless/fades. | Addition of few drops of KMnO4 to ethyne, the purple color changes to colorless/fades. |
| Solubility | They are generally insoluble in polar solvents like water but readily soluble in nonpolar solvents. | They are insoluble in water but readily soluble in organic solvents. | They are insoluble in water but are soluble in organic solvents. |
| Reactivity | Less reactive than alkenes. | More reactive than alkanes. | Alkynes are less reactive than alkene. |
| Acidity | Less acidic than alkene and alkyne. | Less acidic than alkenes. | They are more acidic than alkanes and alkenes. |
| Odor | Odorless | Sweet smell | Odorless and colorless with exception of ethylene which has a slight odor. |
Also Read: Difference Saturated And Unsaturated Hydrocarbons