A covalent bond also referred to as a molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms. These electrons are pairs are referred to as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalence of a full outer shell, corresponding to a stable electronic configuration.
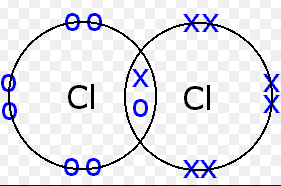
An example of a covalent bond is the Cl-Cl bond in a chloride molecule. Two chloride atoms are attracted to the same pair of electrons. Each chloride atom has seven valence electrons in the third energy level and requires one more electron to form an electron core with an argon electron configuration. Each chloride atom contributes one electron to the bonded pair shared by the two atoms. The remaining six valence electrons of each atom are not involved in bonding.
Covalent bonding does not necessarily require that the two atoms be of the same elements, only that they be of comparable electronegativity. Covalent bonding that entails sharing of electrons over more than two atoms is said to be delocalized.
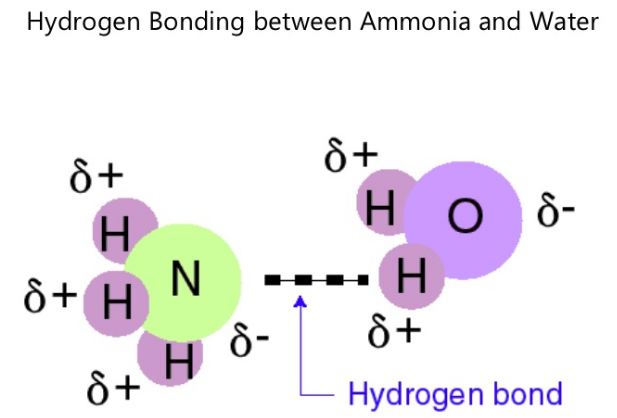
Hydrogen bonds on the other hand, occur when a hydrogen atom undergoes dipole-dipole attraction to an electronegative atom. Usually, hydrogen bonds occur between hydrogen and fluorine, oxygen or nitrogen. Sometimes the bonding is intramolecular or between atoms of a molecule, rather than between atoms of separate molecules (intermolecular).
An example of a molecule that exhibit hydrogen bonding is ammonia (NH3). Hydrogen bonds form between hydrogen of one molecule and nitrogen of another. In the case of ammonia, the bond that forms is very weak because each nitrogen has one lone electron pair.
Read Further: Covalent Vs. Metallic Vs. Ionic Bond
Covalent Bonds Vs. Hydrogen Bonds In Tabular Form
| BASIS OF COMPARISON | COVALENT BONDS | HYDROGEN BONDS |
| Description | Covalent bonds are chemical bonds that are formed due to sharing of electrons between atoms. | Hydrogen bonds are chemical bonds formed by electrostatic attraction forces between hydrogen and an electronegative atom such as O, N, and F. |
| Bonding Constituents | Covalent bond changes the chemical properties of the bonding constituents. | Hydrogen bonding changes the physical properties of bonding constituents. |
| Bond Strength | Covalent bond is a strong bond. Bond energy of covalent bonds is between 100 to 110 kj/mol. | Hydrogen bond is a weak bond. Bond energy of hydrogen bond is between 5 to 50kj/mol. |
| Occurrence | Covalent bonding can occur between polar and non-polar atoms or molecules. | Hydrogen bond formation can occur only in polar molecules. |
| Strength | Covalence will be bigger if the bonded atoms have similar electro-negativity. | Strength of hydrogen bond increases with increase in the electro-negativity difference between hydrogen and the bonded atom. |
| Category of Bond | Covalent bond can be categorized as a primary bond. | Hydrogen bond can be categorized as a secondary bond. |
| Kind Of Bond | Covalent bond’s nature is intra-molecular bond. | The nature of hydrogen bond is inter-molecular bond (and not intramolecular). |
| Bond Formation | Covalent bonds can only be formed between atoms with suitable electron valence. | Hydrogen bond can occur only between hydrogen and an electronegative atom. |
| Atom’s Outer Ortbitals | Covalent bond formation fulfills the valence of the outer orbitals in an atom. | Hydrogen bond formation will not fulfill the valence of atom’s outer orbital. |
| Examples | Example of covalent bond is the bond between hydrogen and oxygen in water molecule. | Example of hydrogen bond: interaction between two strands of DNA and interaction between water molecules and ice. |
What Are Some Of The Similarities of Hydrogen & Covalent Bonds?
- Both covalent and hydrogen bonds are forms of intermolecular forces.
- Covalent and hydrogen bonds are types of chemical bonds.
- Both covalent and hydrogen bonds occur between two atoms.
- Both type of bonds (covalent & hydrogen) act as a glue between two atoms.