Polymerization describes the formation of large molecules (Polymers) with repeating structure from small molecules (monomers). There are two types of polymerization reactions, additional and condensation.
Additional Polymerization
Additional polymerization is a polymerization in which the growth of polymer chains proceeds by addition reactions between molecules of any size. It involves the linking of monomers with double bonds. The double bond of one monomer breaks and links onto the neighboring monomer. This process continues to form polymers, some of which can be many thousands of monomers long.
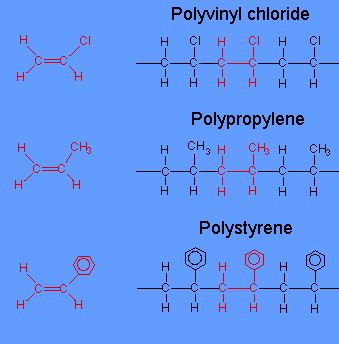
What You Need To Know About Additional Polymerization
- Additional polymerization involves polymers which are formed by the combination of alkene monomers to produce a single huge molecule only.
- Additional polymer is made of only one kind of monomer.
- Typical examples of additional polymerization are the formation of PolyVinyl Chloride (PVC) from Vinyl Chloride or formation of Polyethylene from Ethylene.
- Radical initiators, Lewis acid or bases are used as catalysts in this process. Such reactions are normally catalyzed by peroxides and acids at a pressure of about 1000 atm.
- Additional polymerization does not produce any by-product.
- In additional polymerization, monomer must have at least a double bond or triple bond.
- Additional polymerization reaction results in higher molecular weight polymers at once.
- Additional polymerization produces thermoplastics.
- As the end product, additional polymerization produces higher molecular weight polymers.
- In additional polymerization, longer reaction time has a small effect on the molecular weight of the polymer. Though higher yields are realized due to longer reaction time.
- Additional condensation results in what is known as homo-chain polymers.
- Additional polymerization produces polymers that are non-biodegradable and easy to recycle.
Condensation Polymerization
A condensation polymerization is a form of step-growth polymerization. Small molecules react with each other to form larger structural units while releasing smaller molecules as a byproduct, such as water, HCl or methanol. A good example of polymerization reaction is the esterification of carboxylic acids with alcohols.
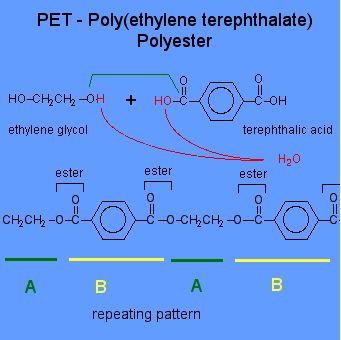

What You Need To Know About Condensation Polymerization
- Condensation polymerization involves polymers which are formed by the combination of monomers with the elimination of simple molecules like water (H2O) or ammonia (NH3).
- A condensation polymer consists of two different kinds of monomers.
- Typical examples of condensation polymers are Polyesters, nylon, Bakelite and Polyamides.
- Mineral acids and bases are normally employed as catalysts in this process.
- In condensation polymerization, HCl, CH3OH, NH3 are usually produced as by-products.
- In condensation polymerization, monomer must have at least two similar or different functional groups.
- Condensation polymerization reaction results in lower molecular weight polymer in the beginning, however, molecular weight of the polymer increases steadily as the reaction progresses.
- Condensation polymerization produces thermosetting plastics (Thermosets).
- Condensation polymerization produces low molecular weight polymers as its end products.
- In condensation polymerization, longer reaction time results in higher molecular weight of the polymers.
- Condensation polymerization result in what is known as hetero-chain polymers.
- Condensation polymerization produces polymers that are biodegradable and easy to recycle.
Also Read: Difference Between Amine And Amide
Additional Vs Condensation Polymerization In Tabular Form
| BASIS OF COMPARISON | ADDITIONAL POLYMERIZATION | CONDENSATION POLYMERIZATION |
| Description | Additional polymerization involves polymers which are formed by the combination of alkene monomers to produce a single huge molecule only. | Condensation polymerization involves polymers which are formed by the combination of monomers with the elimination of simple molecules like water (H2O) or ammonia (NH3). |
| Number Of Monomer | Additional polymer is made of only one kind of monomer. | Additional polymer is made of only one kind of monomer. |
| Examples | Formation of PolyVinyl Chloride (PVC) from Vinyl Chloride or formation of Polyethylene from Ethylene. | Polyesters Nylon Bakelite Polyamides. |
| Catalyst | Radical initiators, Lewis acid or bases are used as catalysts in this process. Such reactions are normally catalyzed by peroxides and acids at a pressure of about 1000 atm. | Mineral acids and bases are normally employed as catalysts in this process. |
| Time | Very fast reaction. | It is a very slow reaction, it takes hours to complete. |
| By-Products | Does not produce any by-product. | HCl, CH3OH, NH3 are usually produced as by-products. |
| Monomer | Monomer must have at least a double bond or triple bond. | Monomer must have at least two similar or different functional groups. |
| Reaction &Molecular Weight | Reaction results in higher molecular weight polymers at once. | Reaction results in lower molecular weight polymer in the beginning, however, molecular weight of the polymer increases steadily as the reaction progresses. |
| Products | Produces thermoplastics. | Produces thermosetting plastics (Thermosets). |
| Molecular Weight | As the end product, additional polymerization produces higher molecular weight polymers. | It produces low molecular weight polymers as its end products. |
| Reaction Time | Longer reaction time has a small effect on the molecular weight of the polymer. Though higher yields are realized due to longer reaction time. | Longer reaction time results in higher molecular weight of the polymers. |
| Type Of Polymer Produced | Results in what is known as homo-chain polymers | Result in what is known as hetero-chain polymers. |
| End Products | Produces polymers that are non-biodegradable and easy to recycle. | Produces polymers that are biodegradable and easy to recycle. |
Similarities Between Additional polymerization And Condensation Polymerization
- Both additional and condensation polymerization are two major processes of producing a polymer compound.
- Both additional and condensation polymerization are catalyzed reactions.
- Both are endothermic reactions. The reaction require heat from an external source.
- Both additional and condensation polymerization entail formation of large molecules (Polymers) with repeating structure from small molecules (monomers).
- End products of both additional and condensation polymerization can be easily deformed or bunched together by heat.
Comments are closed.