Objective
Seliwanoff’s test is a biochemical test devised by the Russian chemist Theodore Seliwanoff in 1887. The main objective of Seliwanoff’s Test is to differentiate between aldoses and ketoses based on their reaction with Seliwanoff’s reagent. Aldoses, which have a terminal aldehyde group, do not give a strong positive reaction or give a much slower one compared to ketoses, which have a ketone group.
In other words, It provides a qualitative assessment of the sugar composition in a given solution. By observing the color change (or lack thereof) upon addition of Seliwanoff’s reagent, one can determine whether the sugar being tested is predominantly a ketose or an aldose.
Principle
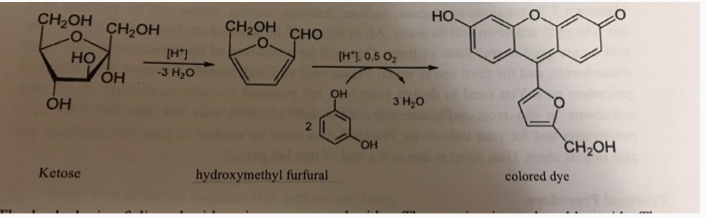
The principle behind Seliwanoff’s Test lies in the specific reaction between the sugar being tested and a specialized reagent known as Seliwanoff’s reagent.
The reagent itself consists of resorcinol, a colorless organic compound, dissolved in an acidic solution. When heated together with a sugar sample, such as fructose or glucose, the solution undergoes a color change if a ketose is present.
The acid hydrolysis of polysaccharide and oligosaccharide ketoses yields simpler sugars followed by furfural. The dehydrated ketose then reacts with two equivalents of resorcinol in a series of condensation reactions to produce a molecule with a deep cherry red color.
The ketone group in ketoses, such as fructose, is more reactive than the aldehyde group present in aldoses, such as glucose. When the ketone group reacts with the acidic conditions provided by Seliwanoff’s reagent and heat, it undergoes dehydration to form furfural compounds. These furfural derivatives then further react with the resorcinol in the reagent, leading to the development of a distinct red color in the solution.
Aldoses do not give a strong positive reaction with Seliwanoff’s reagent or exhibit a much slower reaction rate. This is because aldoses contain a terminal aldehyde group, which is less reactive under the conditions of the test compared to the ketone group present in ketoses.
In other words, When Seliwanoff reagent is added to a solution containing ketoses, a red color is formed rapidly indicating a positive test. When added to a solution containing Aldoses, a slower forming light pink is observed instead.
Materials
- Test solution: 5% Glucose, 5% Sucrose, 5% Fructose
- Seliwanoff’s reagent (0.5% resorcinol in 3N HCl)
- Water bath
- Pipettes
- Dry test tubes
Procedure
- Mix 0.5 grams of resorcinol with 100 mL of 3N hydrochloric acid (HCl). Stir the solution until the resorcinol is completely dissolved. This forms the Seliwanoff’s reagent. Ensure the reagent is freshly prepared for accurate results.
- Prepare separate test solutions containing 5% glucose, 5% sucrose, and 5% fructose. Dissolve 5 grams of each sugar in 100 mL of distilled water to make the respective solutions. Label each solution accordingly.
- Fill a water bath with water and heat it to approximately 50-60°C. The water bath will be used to heat the test tubes containing the sugar solutions during the test.
- Label dry test tubes with the names of the respective sugar solutions to be tested (glucose, sucrose, fructose). Use a marker to ensure clear labeling. Place the test tubes in the water bath to equilibrate to the bath temperature.
- Using pipettes, transfer approximately 2 mL of each sugar solution into the labeled test tubes. Ensure each test tube contains the correct sugar solution and that the volumes are consistent.
- To each test tube, add 5-10 drops of freshly prepared Seliwanoff’s reagent using a dropper or pipette. Mix the contents of each test tube gently by swirling.
- Place the test tubes containing the sugar solutions and Seliwanoff’s reagent into the water bath. Allow the tubes to heat for about 5 minutes, ensuring the temperature remains constant around 50-60°C throughout the duration of the test.
- After 5 minutes, carefully remove the test tubes from the water bath. Observe each test tube for any color change.
- Note: If the reaction is allowed for longer time, Aldoses also produce positive results.
Seliwanoff’s Test Result Interpretation

- Positive Seliwanoff’s Test: If the color changes to red, then your result is positive and keto sugar (Fructose and Sucrose) is present inside the solution.
- Negative Seliwanoff’s Test: If no red color appears or if a faint pink color appears, you’re your result is negative and Aldose sugar (Glucose) is present in the solution.
Limitations of Seliwanoff’s Test
- Certain compounds or impurities in the sample solution may interfere with the reaction between the sugar and Seliwanoff’s reagent, leading to inaccurate results.
- The reaction between the sugar and Seliwanoff’s reagent requires heating for several minutes. This prolonged reaction time may be inconvenient for rapid testing or high-throughput analysis scenarios.
- While Seliwanoff’s Test is valuable for qualitative analysis of sugar solutions, it may not be suitable for quantitative analysis or for detecting sugars in complex matrices such as biological samples or food extracts.
- Interpretation of the test results may be subjective, particularly when the color change is subtle.
- The test may lack sensitivity, especially when the concentration of the ketose in the solution is low.