Objective
Molisch’s test is a sensitive chemical test, named after Australian Botanist Hans Molisch. It is a chemical test used to detect the presence of carbohydrates, specifically the presence of any form of carbohydrate that can be hydrolyzed to form simple sugars, such as glucose.
The objective of Molisch’s test is to identify the presence of carbohydrates in a given sample by observing the formation of a purple-colored ring when the sample is treated with α-naphthol and concentrated sulfuric acid. This purple ring formation indicates the presence of carbohydrates in the sample.
Principle
Molisch’s test entails the addition of Molisch’s reagent (a solution of α-naphthol in ethanol) to the analyte and the subsequent addition of a few drops of concentrated sulphuric acid (H2SO4) to the mixture.
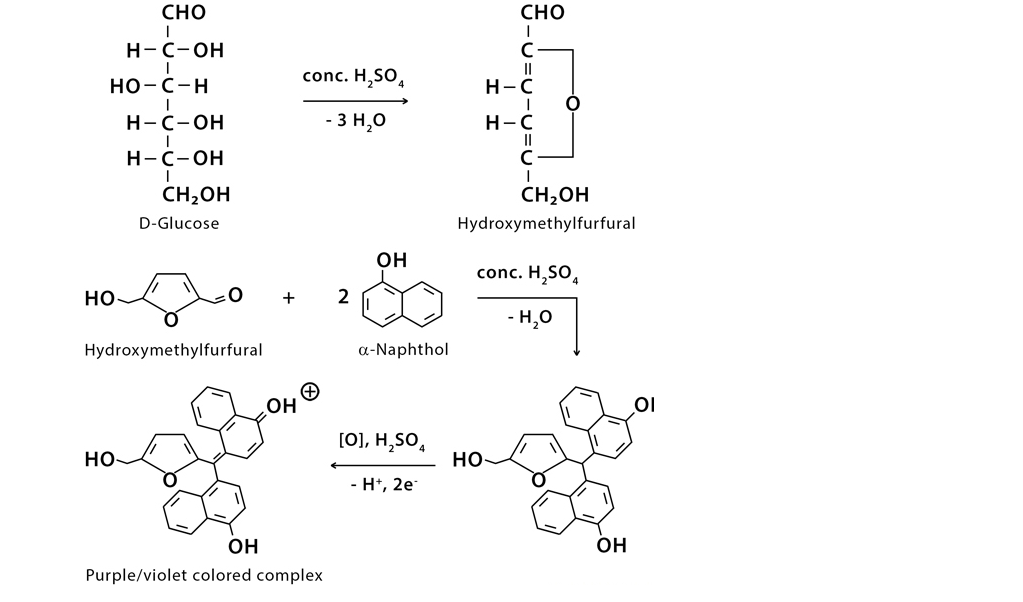
Molisch’s test is based on the principle that when monosaccharides are treated with concentrated sulphuric acid (H2SO4) or concentrated hydrochloric acid (HCl), -OH group of sugar are removed in the form of water resulting in the formation of an aldehyde. These products react with sulphonated α-naphthol to give a purple (reddish-purple) colored complex.
A positive reaction for Molisch’s test is given by almost all carbohydrates except tetroses and trioses. It is also important to note that some glycoproteins and nucleic acids give positive results for this test (since they tend to undergo hydrolysis when exposed to strong mineral acids and form monosaccharides).
Molisch’s test Reaction
Reagents And Apparatus
- Sample solution containing the carbohydrate to be tested
- Molisch’s reagent: α-naphthol solution (5% w/v) in ethanol
- Concentrated sulfuric acid (H₂SO₄)
- Test tubes
- Test tube rack
- Water bath or heating source
- Pipettes
Procedure
Procedure:
- Take a small amount of the sample solution (liquid or dissolved solid) in a clean test tube. The amount of sample used can vary depending on the concentration of carbohydrates expected in the sample.
- Add 2-3 drops of α-naphthol solution (5% w/v) to the sample in the test tube. Make sure to mix the contents of the test tube thoroughly by swirling.
- Now, carefully add concentrated sulfuric acid (H₂SO₄) along the sides of the test tube to form a layer on top of the sample. Be cautious while adding sulfuric acid, as it is highly corrosive and can cause burns. Tilt the test tube slightly and let the acid flow down the side of the tube to form a layer without mixing with the sample.
- Observe the formation of a purple-colored ring at the interface between the sample solution and the concentrated sulfuric acid layer. This ring formation indicates the presence of carbohydrates in the sample.
- If necessary, gently heat the test tube in a water bath or using a suitable heating source for a few minutes to enhance the reaction. However, be cautious not to overheat the mixture to avoid decomposition of carbohydrates or excessive charring.
- Record your observations. The presence of a purple ring confirms the presence of carbohydrates in the sample.
- Dispose of the contents of the test tube properly according to laboratory waste disposal guidelines.
Precaution
Precautions:
- Handle concentrated sulfuric acid with extreme caution to avoid accidents.
- Use proper protective gear such as gloves and goggles when working with sulfuric acid.
- Ensure the test tubes and other glassware are clean and dry before use to prevent contamination.
- Dispose of chemical waste properly according to laboratory safety protocols.
Results Interpretation
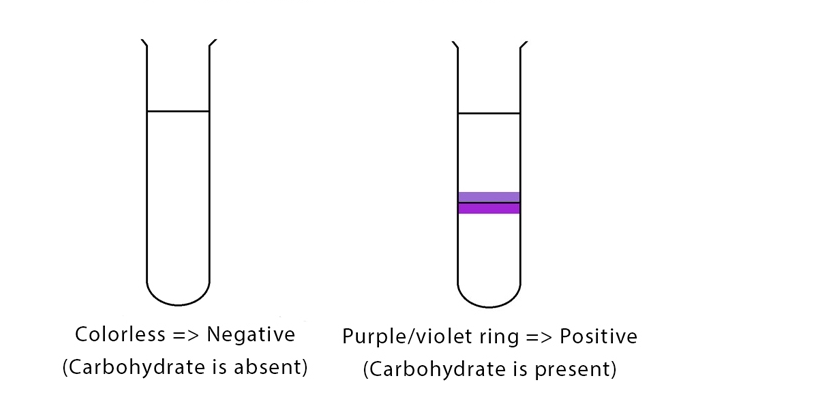
Positive Molisch’s Test: The formation of a purple ring at the layer formed by the concentrated acid is a positive indicator for Molisch’s test.
Negative Molisch’s Test: If no purple or reddish-purple color forms, the given analyte does not contain any carbohydrate.
Limitations of Molisch’s Test
- Molisch’s test can detect a wide range of carbohydrates, including monosaccharides, disaccharides, and polysaccharides. However, it does not distinguish between different types of carbohydrates or provide information about their specific structures.
- Molisch’s test involves the use of concentrated sulfuric acid, which can lead to the degradation of some carbohydrates, especially if the test is carried out under harsh conditions or for an extended period. This degradation can result in the loss of carbohydrate content and affect the accuracy of the test.
- The interpretation of Molisch’s test results relies on visual observation of color changes, particularly the formation of a purple ring. The interpretation of color changes can be subjective and may vary depending on factors such as lighting conditions and individual perception.
- Certain compounds other than carbohydrates, such as phenols and some proteins, can also give a positive result in Molisch’s test. This can lead to false-positive results and misinterpretation of the test outcome.