Principle Of Iodine Test For Starch
The starch-iodide complex as charge is transferred between the starch and iodide ions (tri-iodide or pentaiodide). The transfer of the charge between the starch and the iodide ion changes the spacing between energy levels/orbitals. This change results in the starch-iodide complex absorbing light at different wavelength resulting in an intense color (blue black).
The intensity of the color decreases with increasing temperature and with the presence of water-miscible organic solvents such as ethanol. The test cannot be performed at very low PH due to the hydrolysis of the starch under these conditions.
Iodine test is used to test for the presence of starch in any given food sample. The test can be performed for both the liquid and solid food samples.
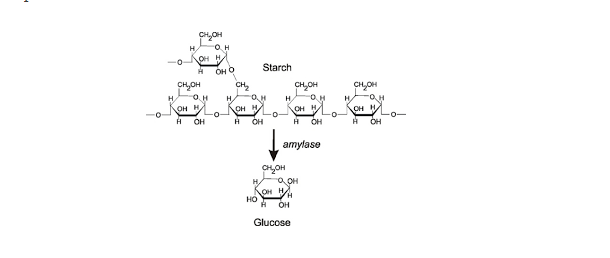
Reagents And Apparatus
- Test substance: This could be a food sample, a plant extract, or any other material you suspect contains starch.
- Iodine solution: You can buy iodine solution at a pharmacy or chemical supply store. It is usually a solution of iodine and potassium iodide (KI) in water, and it appears brownish in color.
- Pipette or dropper: To accurately measure and transfer the iodine solution.
- Test tubes or small containers: To hold the test substance and iodine solution.
- Bunsen burner or hot plate (optional): If you are testing a solid substance like a potato slice, you may need heat to help release the starch for the test.
Iodine Test Procedure
- If you are testing a solid substance, such as a piece of potato or bread, you should start by creating a starch solution. To do this, cut or grind the solid material into small pieces and mix it with a small amount of distilled water. Heat the mixture slightly to help dissolve any starch. Alternatively, you can use a liquid substance, like a plant extract or a food sample, without the need for additional preparation.
- Transfer a small amount of the test substance (solid or liquid) into a test tube or a small container.
- Using a pipette or dropper, add a few drops of iodine solution to the test substance. It’s important to add the iodine solution in small increments to avoid excessive contamination of the solution.
- Observe the color change in the mixture.
- Record your observations, including the color change and any other relevant details.
- If you want to confirm the absence of starch, you can perform a control test by adding a few drops of iodine solution to a separate container with a known starch-free substance (e.g., distilled water) as a comparison.
Precautions
- Use test-tube holder for holding the test tubes and keep the mouth of the test tube away from yourself while heating.
- Use dry-clean test tubes
- Do not use too much of iodine
Result Interpretation
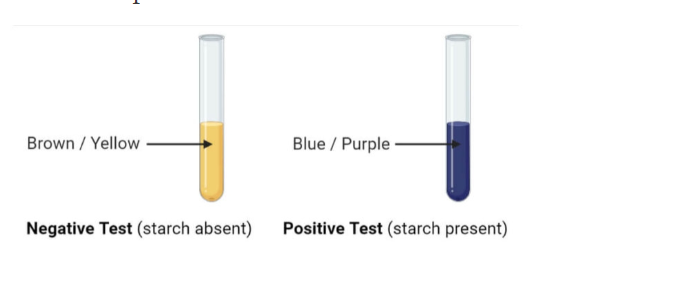
- Positive Result: The color of the solution changes to blue-black on addition of iodine. This indicates that starch is present in the solution.
- Negative Result: No observable color change on addition of iodine solution. This indicates that starch is absent in the solution.
Limitation of Iodine Test
- The iodine test is primarily specific to starch. It may not detect other polysaccharides or carbohydrates with different structures, such as glycogen or cellulose. Therefore, it’s essential to recognize that a positive iodine test indicates the presence of starch but not necessarily other types of carbohydrates.
- The iodine test can detect the presence of starch but may not be highly sensitive in identifying trace amounts. It’s more suited for qualitative analysis (presence or absence) than for quantitative measurements.
- Certain substances, like some dextrins and glycogen, may also produce a positive iodine test because they have similar structures to starch. In some cases, this can lead to false-positive results.
- The color change reaction with iodine solution may take some time to develop fully. It’s important to allow sufficient time for the reaction to occur before interpreting the results accurately.
- Substances in the test sample that can react with iodine or the iodine solution itself can interfere with the test results. This can lead to inaccurate readings. Therefore, it’s important to use distilled water and clean containers to minimize interference.
- The temperature at which the test is conducted can influence the results. Higher temperatures may accelerate the reaction, but extreme heat can denature the starch and affect the outcome.
- The iodine test provides limited information about the type or source of starch present. It cannot differentiate between starch from different plant sources, for example.