Molecular geometry in chemistry is the three-dimensional arrangement of atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom.
Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism as well as the biological activity. Molecular geometries take into account the number of atoms and the number of lone pair electrons. Common molecular geometries include:
- Linear
- Trigonal
- Tetrahedral
- Octahedral
Molecular geometries are determined by the Valence-shell electron repulsion (VSEPR) theory. This is a theory that proposes the geometry of a molecule based on minimizing the repulsion between electron pairs. This model is useful for most compounds with a central atom. A table of geometries using the VSEPR theory can facilitate drawing and understanding molecules.
Electrons, whether bonded or in lone pairs will repel each other and they arrange around a central atom in a way that minimizes this repulsion and maximizes the distance between them. Lone pairs of electrons will repel stronger than bonded ones and this will alter the bonded angles in the molecular geometry, making the angles slightly smaller.
Trigonal Planar
Trigonal planar is a molecular shape that results when there are three bonds and no lone pairs around the central atom in the molecule. In an ideal Trigonal planar species, all three ligands are identical and the pairs are arranged along the central atom’s equator, with 120o bond angles between them. Molecules with a Trigonal planar electron pair geometries have sp2d hybridization at the central atom.
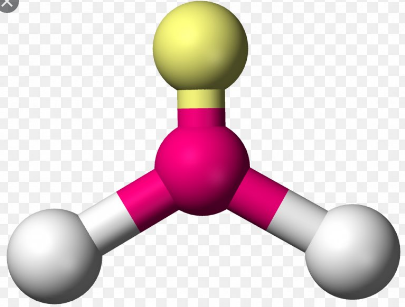
Examples of molecules with Trigonal planar geometry include:
- Boron trifluoride (BF3)
- Formaldehyde (H2CO)
- Phosgene (COCl2)
- Sulfur trioxide (SO3)
Ions with Trigonal planar geometry include:
- Nitrate (NO3 -)
- Carbonate (CO3 2- )
- Guanidinium (C(NH2 )3 + )
What You Need To Know About Trigonal Planar
- Trigonal planar geometry is shown by molecules with four atoms. There is one central atom and the other three atoms (peripheral atoms) are connected to the central atom in a way that they are in the corners of a triangle.
- In Trigonal planar, there is absence of lone pair electrons in the central atom.
- Molecules with the Trigonal planar shape are triangular and in one plane or flat surface.
- A molecule with an ideal Trigonal planar geometry has an angle of 120o between the peripheral atoms.
- Examples of atoms that show Trigonal planar geometry include Boron trifluoride (BF), COCL2, carbonates, sulfates etc.
- In Trigonal planar, there is only bond-bond repulsion.
Trigonal Pyramidal
Trigonal pyramidal is a molecular shape that results when there are three bonds and one lone pair on the central atom in the molecule. In organic chemistry, molecules which have a Trigonal pyramidal geometry are sometimes described as sp3. Ammonia (NH3) is a Trigonal pyramidal molecule. It has three hydrogen atoms and the unshared pair of electrons attached to the nitrogen atom.
The three hydrogen atoms are repelled by the electron lone pair in a way that the geometry is distorted to a Trigonal pyramid, thus, the three hydrogen atoms and the lone electron pair are as far apart as possible at nearly 109o bond angle.
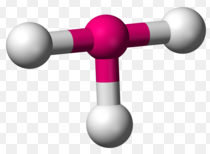
Examples of atoms that show Trigonal pyramidal geometry include:
- Ammonia (NH3)
- Chlorate ion (CIO3 – )
- Sulfite ion (SO3 2- )
- Pnictogen hydrides (XH3)
- Xenon trioxide (XeO3)
What You Need To Know About Trigonal Pyramidal
- Trigonal pyramidal geometry is shown by molecules having four atoms or ligands. Central atoms will be at the apex and three other atoms or ligands will be at one base, where they are in the three corners of a triangle.
- In Trigonal pyramidal, there is presence of one lone pair electrons at the central atom.
- Atoms in Trigonal pyramidal are not in one plane.
- In Trigonal pyramidal, the bonded three atoms and the lone pair electron will be as far apart as possible due to bond repulsion. The angle between the atoms will be less than the angle of a tetrahedron (109o). Usually, the angle in a Trigonal pyramidal is around 107o.
- Examples of atoms that show Trigonal pyramidal geometry include Ammonia, chlorate ion and sulfite ion.
- In Trigonal pyramidal, there is bond-bond, and bond-lone pair repulsion.
Also Read: Difference Between SN1 And SN2 Reaction
Difference Between Trigonal Planar And Trigonal Pyramidal In Tabular Form
| BASIS OF COMPARISON | TRIGONAL PLANAR | TRIGONAL PYRAMIDAL |
| Description | Trigonal planar geometry is shown by molecules with four atoms. There is one central atom and the other three atoms (peripheral atoms) are connected to the central atom in a way that they are in the corners of a triangle. | Trigonal pyramidal geometry is shown by molecules having four atoms or ligands. Central atoms will be at the apex and three other atoms or ligands will be at one base, where they are in the three corners of a triangle. |
| Lone Pair Electrons | There is absence of lone pair electrons in the central atom. | There is presence of one lone pair electrons at the central atom. |
| One Plane | Molecules with the Trigonal planar shape are triangular and in one plane or flat surface. | Atoms in Trigonal pyramidal are not in one plane. |
| Bond Angle | A molecule with an ideal Trigonal planar geometry has an angle of 120o between the peripheral atoms. | The bond angle in a Trigonal pyramidal is around 107o. |
| Examples | Boron trifluoride (BF), COCL2, carbonates, sulfates etc. | Ammonia, chlorate ion and sulfite ion. |
| Bond Repulsion | There is only bond-bond repulsion. | There is bond-bond, and bond-lone pair repulsion. |