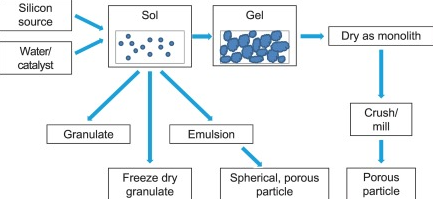
A sol is a colloidal solution suspension of very small solid particles in a continuous liquid medium. Sols are quite stable and show the Tyndall Effect. Tyndall Effect is the scattering of visible light by colloidal particles. Artificial sols may be prepared by dispersion or condensation. Dispersion techniques include grinding solids to colloidal dimensions by ball milling and Bredig’s arc method (whereby an arc is struck between electrodes under the surface of water containing some stabilizing agents). A sol generally has a liquid as the dispersing medium and solid as a dispersed phase.
Examples of sols include:
- Mud
- Milk of magnesia
- Paint
- Cell fluids
- Blood
- Pigmented ink
Gels are colloids in which the liquid medium has become viscous enough to behave more or less as a solid. A gel can also be described as dispersion of molecules of a liquid within a solid in which liquid particles are dispersed in solid medium. A gel may be notably elastic and jelly-like or quite solid and rigid.
Gels consist of a solid three-dimensional network that span the volume of a liquid medium and ensnares it through surface tension effects. Both by weight and volume, gels are mostly fluid in composition and thus exhibit densities similar to those of their constituent liquids.
Examples of Gel include:
- Fruit jelly
- Cooked gelatin jelly
- Fog
- Mist
- Ice cloud
- Hair sprays.
Gel Vs. Sol In Tabular Form
| BASIS OF COMPARISON | SOL | GEL |
| Description | A sol is a colloid made out of very small particles in a continuous liquid medium. Sols are stable and show the Tyndall effect. | A gel is a complex fluid consisting of two or more phases typically composed of a solid dispersed in a liquid. |
| Alternative Description | Sol is the liquid state of a colloid solution. | Gel can be described as solid or semi-solid (jelly-like) stage of a colloidal solution. |
| Conversion | The sol can be converted to gel by cooling. | The gel can be converted to sol by heating. |
| Dehydration | Dehydration of sol is relatively very easy. | Dehydration of gel is not possible. |
| Viscosity | Sol has a low viscosity. | The gel has a high viscosity. |
| Classification | Sol is usually classified further to lyophobic and lyophilic. | Gel does not have a further classification. |
| Definite Structure | The sol does not possess a definite structure. | The gel has a honeycomb-like structure. |
| Dispersion Medium | The dispersion medium of the sol may be water (hydrosol) or alcohol (alcosol). | The dispersion medium of the gel will be hydrated colloid particles. |
| Examples | Blood, pigmented ink, cell fluids, paint, milk of magnesium and mud. | Fruit jelly, cooked gelatin jelly, fog, mist, ice cloud, hair sprays. |