Flames burn by combining their own carbon molecules with oxygen molecules in the air to form carbon dioxide; in this regard heat flames are classified as either luminous or non-luminous flames. The terms “luminous” and “non-luminous” refer to objects based on their ability to emit light.
Luminous flames do not get enough oxygen to turn all the carbon that is being burnt into carbon dioxide. Some of this excess carbon produces soot. Non-luminous flames have unlimited access to oxygen and therefore, are able to combine all their carbon with oxygen, they burn more efficiently and are much hotter than luminous flames.
In a Bunsen burner for example, non-luminous flames are formed when the air-hole is opened luminous flames are produce when the air-hole is closed.
Due to availability of different levels of oxygen, luminous flame burns with a visibly bright yellow color whereas non-luminous flames burn with light blue color.
More importantly, non-luminous flames do not produce soot, burn steadily and produce more heat or are much hotter when compared to non-luminous flames. In this regard, non-luminous flames are most preferably used in experiments (laboratory operations).
Luminous Flame has three main regions:
- The top yellow region where there is incomplete combustion/burning.
- The region of unburnt gas, below the yellow region where the gas does not burn.
- Blue region on the sides of region of unburnt gas where there is complete burning.
Non-luminous flame has four main regions:
- The top colorless region
- Blue region just below where there is complete burning. It is the hottest region.
- Green region surrounded by the blue region where there is complete burning.
- The region of unburnt gas at the innermost surrounded by green and blue regions. No burning takes place here.
Characteristics Of Luminous Flames
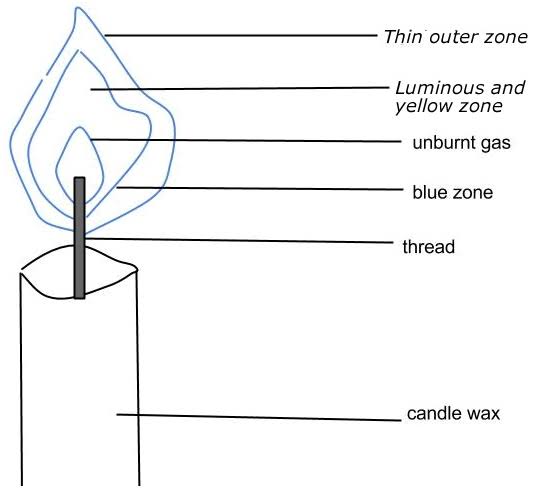
- Luminous flames are bright yellow in color.
- Luminous flames are sooty (produce soot)
- Flames are not steady (do not burn steadily).
- Luminous flames are not very hot (produce less heat).
- Luminous flame produces more light.
- Luminous flames do not burn more efficiently. Luminous flames do not get enough oxygen to turn all the carbon that is being burnt into carbon dioxide.
- Luminous flames have limited access to oxygen.
- Luminous flames are wavy and brightly visible
- Luminous flames are not used in experiments (not best for laboratory operations) because it is wavy and sooty in nature.
- In a Bunsen burner, luminous flame is formed when the air-hole is closed.
- Examples of luminous flames include burning wood, candles, Olympic cauldron etc.
Characteristics Of Non-Luminous Flames
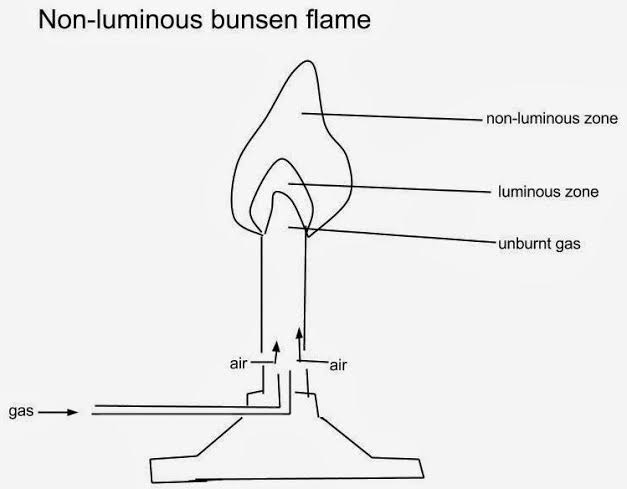
- Non-luminous flame is light blue in color.
- Non-luminous flames are not sooty (does not produce soot).
- Flames are steady.
- Non-luminous flames are very hot (Produce more heat).
- Non-luminous flames produce little light.
- Non-luminous flames burn more efficiently because they are able to combine all their carbon with oxygen.
- Non-luminous flames have unlimited access to oxygen.
- Non-luminous flames are hardly visible and less wavy.
- Non-Luminous flames are most preferably used in experiments (laboratory operations) because they are hot, not sooty, and less wavy and hence easy to control.
- In a Bunsen burner, non-luminous flames are formed when the air-hole is opened.
- Examples of non-luminous flames include flames of a Bunsen burner when the air-hole is closed, acetylene torches etc.
Also Read: Difference Between Luminous And Non-Luminous Objects
Luminous vs Non-luminous Flame: Key Differences
| BASIS OF COMPARISON | LUMINOUS FLAMES | NON-LUMINOUS FLAMES |
| Color | Luminous flame is bright yellow in color. | Non-luminous flame is light blue in color. |
| Soot | Flames are sooty (produces soot). | Flames are not sooty (Does Not produces soot). |
| Burning Stability | Flames are not steady. | Flames are steady |
| Heat Intensity | Flames are not very hot (produce less heat). | Flames are very hot (Produce more heat). |
| Light | Flames produce more light. | Flames produce little light. |
| Burning Efficiency | Flames do not burn more efficiently. Luminous flames do not get enough oxygen to turn all the carbon that is being burnt into carbon dioxide. | Flames burn more efficiently because they are able to combine all their carbon with oxygen. |
| Oxygen Access | Flames have limited access to oxygen. | Flames have unlimited access to oxygen. |
| Visibility | Flames are wavy and brightly visible | Flames are hardly visible and less wavy. |
| Experimental Preference | Flames are not used in experiments because it is wavy and sooty in nature. | Flames are most preferably used in experiments because they are hot, not sooty, and less wavy and hence easy to control. |
| Bunsen Burner | In a Bunsen burner, luminous flame is formed when the air-hole is closed. | In a Bunsen burner, non-luminous flames are formed when the air-hole is open. |
| Examples | Examples of luminous flames include burning wood, candles, Olympic cauldron etc. | Examples of non-luminous flames include flames of a Bunsen burner when the air-hole is closed, acetylene torches etc. |
Key Takeaway
Luminous flames emit visible light due to the presence of solid particles and are associated with incomplete combustion, while non-luminous flames do not emit visible light, resulting from complete combustion and higher temperatures. Non-luminous flames are very hot in nature than luminous flames.