What Are Isotopes?
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have almost the same chemical properties, they have different atomic masses and physical properties.
The number of protons within the atom’s nucleus is called atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom’s mass number, and each isotope of a given element has a different mass number.
For example, carbon-12, carbon-13, and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13, and 14, respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons so that the neutron numbers of these isotopes are 6, 7, and 8 respectively.
What You Need To Know About Isotopes
- Isotopes are atoms with a different number of neutrons but they have the same number of protons and electrons.
- They are atoms of the same element.
- Isotopes have the same atomic number.
- Isotopes have different atomic mass.
- Isotopes have different numbers of neutrons.
- Isotopes of an element have the same chemical properties due to similarity in arrangement and number of electrons.
- Isotopes occupy same position in the periodic table.
What Are Isobars?
Isobars are atoms (nuclides) of different chemical elements that have the same number of nucleons. Correspondingly, isobars differ in atomic number (or number of protons) but have the same mass number. An example of a series of isobars would be Argon, potassium, and calcium having atoms of the same mass number 40 i.e 18Ar40, 19K40, and 20Ca40. While the nuclei of these nuclides all contain 40 nucleons, they contain varying numbers of protons and neutrons.
18Ar40, 19K40, 20Ca40 where 18, 19 and 20 shown as subscripts are the atomic numbers of the three elements respectively are isobars. Since the atomic numbers are different their chemical properties are also different. The structure of argon, potassium, and calcium are given below.
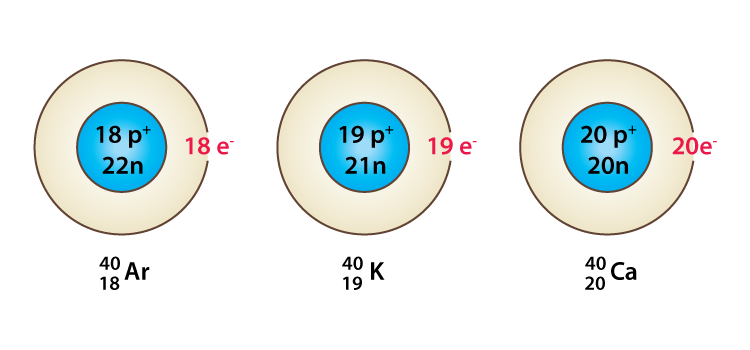
What You Need To know About Isobars
- Isobars are atoms of different chemical elements having same mass but different number of protons and electrons.
- They are atoms of different elements.
- Isobars have different atomic number.
- Isobars have the same atomic mass.
- Isobars have different numbers of neutrons.
- Isobars differ in their chemical properties due to difference in the arrangement of electrons.
- Isobars occupy different position in the periodic table.
Also Read: Difference Between Atoms And Molecules
Difference Between Isotopes And Isobars In Tabular Form
| BASIS OF COMPARISON | ISOTOPES | ISOBARS |
| Description | Isotopes are atoms with a different number of neutrons but they have the same number of protons and electrons. | Isobars are atoms of different chemical elements having same mass but different number of protons and electrons. |
| Nature | They are atoms of the same element. | They are atoms of different elements. |
| Atomic Number | Isotopes have the same atomic number. | Isobars have different atomic number. |
| Atomic Mass | Isotopes have different atomic mass. | Isobars have the same atomic mass. |
| Number Of Neutrons | Isotopes have different numbers of neutrons. | Isobars have different numbers of neutrons. |
| Chemical Properties | Isotopes of an element have the same chemical properties due to similarity in arrangement and number of electrons. | Isobars differ in their chemical properties due to difference in the arrangement of electrons. |
| Periodic Table Position | Isotopes occupy same position in the periodic table. | Isobars occupy different position in the periodic table. |