Daniell Cell
The Daniell cell is a type of electrochemical cell invented in 1836 by John Frederic Daniell, a British chemist and meteorologist and consists of a copper pot filled with a copper (II) sulphate solution, in which is immersed in unglazed earthware container filled with sulphuric acid and a zinc electrode. The Daniell cell can be used to ‘generate electricity’ or store electricity by consuming an electrode.
The redox reaction is the theory behind the Daniell cell. In a Daniell cell, electrons flow from zinc electrode to copper electrode through an external circuit, while metal ions form one half cell to the other through the salt bridge. In this cell, current flows from copper electrode to zinc electrode that is cathode to anode via an external circuit. Here, a salt bridge acts as an electrical contact between the two half cells. It prevents mechanical flow of solution but it provides a free path for the migration of ions, to maintain an electric current through the electrolyte solution. It prevents accumulation of charges.
It is important to note that the Daniell cell is a reversible cell due to the fact that it consists of electrons negative charges traveling around the outer circuit, from the cathode to the anode, while the positive ions travel from the cathode to the anode via the solution. The Daniell cell can be conventionally represented as:
Zn(s) +Cu 2+ (aq)à Zn 2+ (aq) +Cu(s)
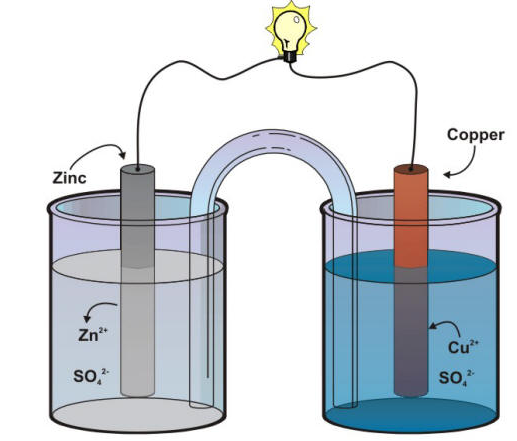
What You Need To Know About Daniell Cell
- Daniell cell is a type of electrochemical cell that is composed of a copper electrode and a zinc electrode immersed in copper (II) sulfate and zinc sulfate respectively.
- Daniell cell uses copper sulfate and zinc sulphate as electrolytes for the completion of the electrochemical cell and redox reaction to take place.
- Daniell cell uses copper cathode electrode for spontaneous redox reactions due to its ability to be an excellent reducing agent.
- In Daniell cell, the anode electrode is composed of zinc electrode as an oxidizing agent for a redox reaction to take place.
Galvanic Cell
A galvanic cell also referred voltaic cell, named after Luigi Galvani or Alessandro Volta respectively, is an electrochemical cell that converts the chemical energy of spontaneous redox reactions into electrical energy. It generally consists of two half cells and a salt bridge. Each half-cell further consists of a metallic electrode dipped into an electrolyte. These two half-cells are connected to a voltmeter and a switch externally with the help of metallic wires.
The working of a galvanic cell is quite simple. It involves a chemical reaction that makes the electric energy available as the end result. During a redox reaction, a galvanic cell utilizes the energy transfer between electrons to convert chemical into electric energy.
In a galvanic cell, when an electrode is exposed to the electrolyte at the electrode-electrolyte interface, have atoms of the metal electrode have a tendency to generate ions in the electrolyte solution leaving behind the electrons at the electrode, thus making the metal electrode negatively charged. At the same time, metal ions in the electrolyte solution have a tendency to deposit on a metal electrode, thus making the electrode positively charged. Out of the two electrodes, the electrode at which oxidation takes place is referred to as anode whereas the electrode at which reduction takes place is referred to as cathode.
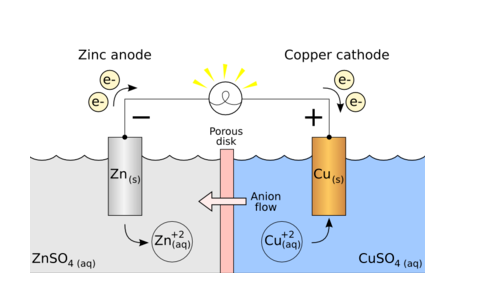
What You Need To Know About Galvanic Cell
- Galvanic cell is a type of electrochemical cell that uses spontaneous redox reaction to generate electrical energy.
- In galvanic cell, the electrolytes used are the salts of metals of each electrode.
- In Galvanic cell, cathode electrode can compose of any metal that can reduce.
- In galvanic cell, the anode is composed of a metal that can be oxidized.
Also Read: Difference Between Electrolytic Cell And Electrochemical Cell
Difference Between Daniell Cell And Galvanic Cell In Tabular Form
| BASIS OF COMPARISON | DANIELL CELL | GALVANIC CELL |
| Description | Daniell cell is a type of electrochemical cell that is composed of a copper electrode and a zinc electrode immersed in copper (II) sulfate and zinc sulfate respectively. | Galvanic cell is a type of electrochemical cell that uses spontaneous redox reaction to generate electrical energy. |
| Electrolyte | Daniell cell uses copper sulfate and zinc sulphate as electrolytes for the completion of the electrochemical cell and redox reaction to take place. | The electrolytes used are the salts of metals of each electrode. |
| Cathode | Daniell cell uses copper cathode electrode for spontaneous redox reactions due to its ability to be an excellent reducing agent. | Cathode electrode can compose of any metal that can reduce. |
| Anode | The anode electrode is composed of zinc electrode as an oxidizing agent for a redox reaction to take place. | The anode is composed of a metal that can be oxidized. |