Amylose and amylopectin are present side by side and interspersed in the starch granule. They have unique chemical and physical properties and their relative proportions influence the overall properties of starches. Amylose forms a colloidal dispersion in hot water whereas amylopectin is completely insoluble.
What Is Amylose?
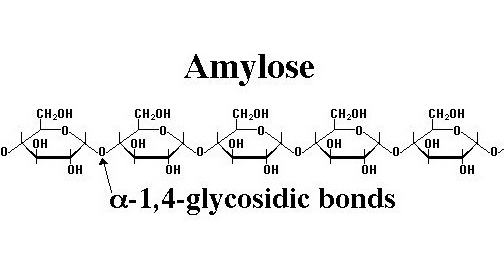
Amylose also referred to as a linear polymer, is one of the two main components of starch. Amylose can be described as a linear polysaccharide in which anhydroglucose units are linked by a-d-1,4 glucosidic bonds. It forms about 20-30% of the starch structure.
They are also good film formers and result in a firm gel when cooked. Amylose molecules may comprise between 200-6000 anhydroglucose units, varying between different starch types. The molecules tend to adopt a natural helica structure. More importantly, amylose is known to interact with iodine, organic alcohols and fatty acids. It gives a blue color in iodine test that helps in distinguishing it from other such components.
Amylose content varies considerably among different starches and genetic modifications have been carried out to obtain starch with amylose contents varying from 1% to 75%. A common source of high amylose starch is a hybrid corn containing over 50% up to 70% amylose. Current due to plant breeding, high amylose rice and potato starches are available.
What You Need To Know About Amylose
- Amylose is an un-branched chain polymer of D-glucose units.
- It gives a dark blue/black color when iodine solution is added.
- Amylose is less soluble in water.
- It does not form a gel when hot water is added.
- Amylose constitutes about 20-30% of the starch.
- Amylose can be hydrolyzed with α amylase and β amylase enzymes completely.
- It has α 1-4 glycosidic linkages.
- Amylose is a straight chain structure.
- Amylose is a great storage system for energy.
- Amylose has 300-several thousand units of glucose.
What Is Amylopectin?
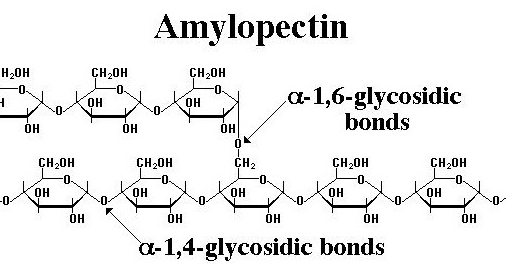
Amylopectin is also a component of starch. It is a water-soluble polysaccharide and highly branched polymer of α-glucose units found in plants. The glucose units are linked in a linear way with α glycosidic bonds in branch chain. Amylopectin is highly branched, being formed of 2000 to 200000 glucose units. Its inner chains are formed of 20-30 glucose subunits.
Amylopectin forms about 80-85% of starch, though the percentage varies depending on the source. For example the content of amylopectin is 100% in medium grain rice, waxy potato starch, waxy corn and glutinous rice; and lower in long-chain rice, amylomaize and russet potatoes.
Amylopectin is an energy source especially in animals. It gives reddish brown color in iodine test and that helps in distinguishing it from others.
What You Need To Know About Amylopectin
- Amylopectin is a branched chain polymer of D-glucose units.
- It gives a reddish brown color when iodine solution is added.
- Amylopectin is more soluble in water.
- It forms a gel when hot water is added to it.
- Amylopectin constitutes about 70-80% of the starch.
- Amylopectin cannot be hydrolyzed with α amylase and β amylase enzymes completely.
- It has a α 1-4 glycosidic linkages and α 1-6 glycosidic linkages.
- Amylopectin is a branched structure.
- Amylopectin only stores a small amount of energy.
- Amylopectin has 2000-200,000 units of glucose.
Amylose vs Amylopectin In Tabular Form
| BASIS OF COMPARISON | AMYLOSE | AMYLOPECTIN |
| Description | Linear polymer of glucose molecules linked by α-1,4-glycosidic bonds | Branched polymer of glucose molecules linked by α-1,4-glycosidic and α-1,6-glycosidic bonds |
| Iodine Test | It gives a dark blue/black color when iodine solution is added. | It gives a reddish brown color when iodine solution is added. |
| Solubility | Insoluble in cold water, forms colloidal suspension when heated | Insoluble in water, swells in water forming colloidal suspension when heated |
| Reaction With Hot Water | It does not form a gel when hot water is added. | It forms a gel when hot water is added to it. |
| Percentage In Starch | Found in smaller quantities in starch (about 20-30%) | Found in larger quantities in starch (about 70-80%) |
| Digestibility | Digestible by amylase enzymes | Digestible by amylase enzymes |
| Hydrolysis | It can be hydrolyzed with α amylase and β amylase enzymes completely. | It cannot be hydrolyzed with α amylase and β amylase enzymes completely. |
| Glycosidic Linkages | It has α 1-4 glycosidic linkages. | It has α 1-4 glycosidic linkages and α 1-6 glycosidic linkages. |
| Glycemic Index | Lower glycemic index compared to amylopectin | Higher glycemic index compared to amylose |
| Energy Content | Mainly for energy storage and slow-release energy source. Found in grains, seeds, and tubers | Provides readily available energy source and quick energy release. Found in grains, seeds, and tubers |
| Units Of Glucose | It has 300-several thousand units of glucose. | It has 2000-200,000 units of glucose. |
Similarities Between Amylose And Amylopectin
- Both amylose and amylopectin are found in starch granules.
- Amylose and amylopectin are both composed of D-glucose units.
- Both amylopectin and amylose are polysaccharide molecules.
- Amylopectin and amylose molecules have α 1-4 glycosidic linkages.
Key Takeaways
Amylose
- Amylose is a linear polymer of glucose molecules linked together by alpha-1,4-glycosidic bonds.
- It is insoluble in cold water but can form a colloidal suspension when heated, due to the disruption of intermolecular forces.
- Amylose is a digestible polysaccharide broken down by enzymes like amylase into maltose, a disaccharide, during the process of digestion.
- It serves as a storage polysaccharide in plants, particularly in starch granules found in seeds, tubers, and other storage organs.
- When amylose molecules are heated and then cooled, they can form a gel network, contributing to the thickening and texture of certain foods like puddings and sauces.
- As a complex carbohydrate, amylose has a lower glycemic index compared to simple sugars, leading to slower glucose release into the bloodstream, which may help in managing blood sugar levels.
Amylopectin
- Amylopectin is a branched polymer of glucose molecules linked together by alpha-1,4-glycosidic bonds and alpha-1,6-glycosidic bonds.
- Unlike amylose, amylopectin has numerous branches due to the presence of alpha-1,6-glycosidic bonds, which occur roughly every 24 to 30 glucose units.
- Amylopectin is insoluble in water but can swell and form a colloidal suspension when heated, contributing to the thickening of starchy foods.
- Amylopectin is also a digestible polysaccharide, broken down into glucose units by amylase enzymes during digestion.
- It is a major component of starch granules in plants, serving as a readily available energy source for the plant and as a storage polysaccharide in organs such as seeds, tubers, and grains.
- While amylose primarily contributes to gel formation in starchy foods, amylopectin also plays a role in thickening and gel formation, albeit to a lesser extent, due to its branched structure.