Description
An antibonding molecular orbital in chemical bonding theory, is a type of molecular orbital (MO) that weakens the chemical bond between two atoms and help to raise the energy of the molecule relative to separate atoms. Such an orbital has one or more nodes in the bonding region between the nuclei. The density of the electrons in the orbital is concentrated outside the bonding region and acts to pull one nucleus away from the other and tends to cause mutual repulsion between the two atoms.
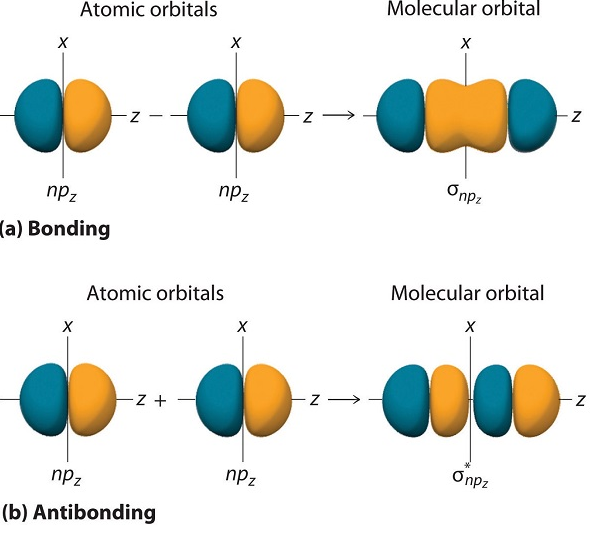
A molecular orbital becomes antibonding when there is less electron density between the two nuclei than there would be if there were no bonding interactions at all. When a molecular orbital changes sign from positive to negative at a nodal plane between two atoms, it is said to be antibonding with respect to those atoms. On molecular orbital diagrams, antibonding orbitals are often labeled with an asterisk (*).
Bonding Molecular Orbital in chemical bonding theory can be described as the attractive interaction between the atomic orbitals of two or more atoms in a molecule. In this theory, electrons are potrayed to move in waves. When more than one of these waves comes close together, the in-phase combination of these waves produces an interaction that leads to a species that is greatly stabilized. The result of the waves’ constructive interference causes the density of the electrons to be found within the binding region, creating a stable bond between the two species.
The Difference
- Antibonding molecular orbitals can be described as orbitals containing electrons outside the region between two atomic nuclei. In contrast, bonding molecular orbitals can be described as type of molecular orbitals that take part in the formation of a chemical bond.
- Antibonding molecular orbitals are formed by out-of-phase combination of atomic orbitals. The combination decreases the electron density in the region between the nuclei of the atoms and increases in the region away from the inter-nuclear region. On the other hand, bonding molecular orbitals are formed by in-phase combination of atomic orbitals. The combination increases the electron density in the region between the nuclei of bonded atoms.
- Antibonding molecular orbitals do not contribute to the shape of the molecule whereas bonding molecular orbitals contribute directly to the shape of the molecule.
- Antibonding molecular orbitals are formed when the lobes of the combining orbitals have opposite sign whereas bonding orbitals are formed when the lobes of the combining orbitals have the same sign.
- The electron density in antibonding molecular orbitals is low whereas the electron density in bonding molecular orbitals is higher.
- The energy of antibonding molecular orbitals is comparatively higher when compared to the energy of bonding molecular orbitals.
- Antibonding molecular orbitals are formed by the subtractive effect of the atomic orbitals whereas the bonding molecular orbitals are formed by the additive effect of atomic orbitals.
- Antibonding molecular orbitals are less stable than both bonding molecular orbitals and parent atomic orbitals. On the other hand, bonding molecular orbitals are more stable than both antibonding molecular orbitals and parent atomic orbitals.
- The electrons in antibonding molecular orbitals do not contribute to the formation of the bond whereas the electrons in bonding molecular orbitals contribute to the formation of a bond.
- The geometry of a molecule does not depend on the spartial arrangement of antibonding molecular orbitals. On the contrary, the geometry of a molecule is represented by the spatial arrangement of bonding molecular orbitals.
- The antibonding molecular orbitals are represented using an asterisk mark (*) while the bonding molecular orbitals are represented without using an asterisk (*).
- When electrons are filled into antibonding molecular orbitals, it destabilizes the bond between two atoms. On the contrary, when electrons are first filled to the bonding molecular orbitals, they stabilize the molecule because they associate less energy than the electron in the parent atom.
ALSO READ: Difference between Orbit and orbitals
Bonding Vs. Antibonding Molecular Orbitals In Tabular Form
| BASIS OF COMPARISON | BONDING MOLECULAR ORBITALS | ANTIBONDING MOLECULAR ORBITALS |
| Description | Bonding molecular orbitals can be described as type of molecular orbitals that take part in the formation of a chemical bond. | Antibonding molecular orbitals can be described as orbitals containing electrons outside the region between two atomic nuclei. |
| Formation | Formed by in-phase combination of atomic orbitals. | Formed by out-of-phase combination of atomic orbitals. |
| Contribution to the Shape of The Molecule | Directly contribute to the shape of the molecule. | Do not contribute to the shape of the molecule. |
| Formed When | Formed when the lobes of the combining orbitals have the same sign. | Formed when the lobes of the combining orbitals have opposite sign. |
| Electron Density | The electron density in bonding molecular orbitals is higher. | The electron density in antibonding molecular orbitals is low. |
| Energy | The energy of bonding molecular orbitals is comparatively lower. | The energy of antibonding molecular orbitals is comparatively higher. |
| Formation Description | Formed by the additive effect of atomic orbitals. | Formed by the subtractive effect of the atomic orbitals. |
| Stability | Bonding molecular orbitals are more stable than both antibonding molecular orbitals and parent atomic orbitals. | Antibonding molecular orbitals are less stable than both bonding molecular orbitals and parent atomic orbitals. |
| Molecular Geometry | The geometry of a molecule is represented by the spatial arrangement of bonding molecular orbitals. | The electrons in antibonding molecular orbitals do not contribute to the formation of the bond. |
| Asterisk Mark | Represented without using an asterisk (*). | Represented using an asterisk mark (*) |
| When Electrons Are Filled | When electrons are first filled to the bonding molecular orbitals, they stabilize the molecule because they associate less energy than the electron in the parent atom. | When electrons are filled into antibonding molecular orbitals, it destabilizes the bond between two atoms. |