Benedict’s test is a simple chemistry test used to detect presence of reducing sugars in a biological sample. It is named after American chemist Stanley Rossister Benedict. Reducing sugars are carbohydrates that can act as reducing agents due to the presence of free aldehyde groups or free ketone groups in their chemical structure. Examples of reducing sugars include: maltose, lactose, galactose, glucose, mannose, fructose etc.
Objective
- To determine the presence or absence of reducing sugar in the solution.
- To determine the glucose concentration in the solution quantitatively.
Principle
The Benedict’s Test is grounded in the principle of detecting reducing sugars through their ability to reduce copper(II) ions to copper(I) ions under alkaline conditions. Benedict’s reagent, composed of copper(II) sulfate (CuSO4) and sodium carbonate (Na2CO3), forms a blue-colored complex of copper(II) ions.
When a solution containing reducing sugars is mixed with Benedict’s reagent and heated, the reducing sugars donate electrons to the copper(II) ions, causing them to be reduced to copper(I) ions. This reduction reaction leads to the formation of insoluble colored precipitates of copper(I) oxide (Cu2O) or copper(II) oxide (CuO), which vary in color from green to yellow to red depending on the amount of reducing sugar present and the specific conditions of the test.
In other words, Benedict’s test is performed by heating the reducing sugar with Benedict‘s reagent. The presence of the alkaline sodium carbonate converts the sugar into a strong reducing agent called enediols. During the reduction reaction, the mixture will change its color from blue to brick-red precipitate due to the formation of cuprous oxide (Cu2O). Copper in its cupric (Cu2+) or copper (I) form is reduced to cuprous (Cu+) or copper (II). The red-colored cuprous oxide is insoluble in water and hence, separated. If the concentration of the sugar is high, then the color becomes more reddish, and the volume of the precipitate increases.
Reagent
Benedict’s reagent is a chemical reagent and complex mixture of sodium carbonate, sodium citrate and copper II sulphate pentahydrate. It is often used in place of Fehling’s solution to detect the presence of reducing sugars. A positive test with Benedict’s reagent is shown by a color change from clear blue to brick-red with a precipitate.
- Test solutions: 5% Glucose, 5% Sucrose
- Benedict’s reagent: CuSO4.5H2O solution with sodium carbonate and sodium citrate.
- Water bath
- Dry bath
- Dry test tubes
- Sodium carbonate (Na2CO3 2-), provides the alkaline conditions which are required for the redox reaction.
- Sodium citrate complexes with the copper II ions so that they do not deteriorate to copper II ions during storage.
- Test tube holder or tongs
- Safety goggles and lab coat (for safety)
Preparation
Procedure of Benedict’s Test
- Ensure that your Benedict’s reagent is freshly prepared. If it has been stored for an extended period, it may not give accurate results.
- Take the liquid or solution you want to test for reducing sugars. If it’s a solid, you may need to dissolve it in water. Make sure the sample is in a liquid form.
- Use a marker to label the test tubes with the sample names or numbers.
- Using a pipette or graduated cylinder, transfer a known volume of the sample into the labeled test tube. The exact volume may vary depending on your specific experiment, but 1-2 mL is sufficient.
- Add an equal volume of Benedict’s reagent to the test tube containing the sample. For example, if you added 1 mL of sample, add 1 mL of Benedict’s reagent.
- Gently swirl or mix the contents of the test tube to ensure thorough mixing of the sample and the reagent.
- Place the test tube in a water bath or heat it over a Bunsen burner flame. The water bath should be at boiling temperature, typically 100°C (212°F). Heat the mixture for about 2-5 minutes.
- While heating, observe any color changes in the solution.
- After heating, carefully remove the test tube from the water bath or Bunsen burner using tongs or a test tube holder. Allow it to cool down for a moment. Then, compare the color of the solution to a color chart to estimate the amount of reducing sugar present.
Observation
When Benedict’s reagent solution and reducing sugars are heated together, the solution changes its color to orange-red/brick red precipitate. The red-colored cuprous oxide is insoluble in water and hence separates out from the solution.
When the concentration of reducing sugar is high in the solution, then the color becomes more intense (reddish) and the volume of the precipitate increases in the test tube making it clearly visible.
Result Interpretation
- Positive Benedict’s Test: Formation of a reddish precipitate within three minutes. Reducing sugars present. Example: Glucose.
- Negative Benedict’s Test: No color change (Remains blue). Reducing sugars absent. Example: Sucrose.
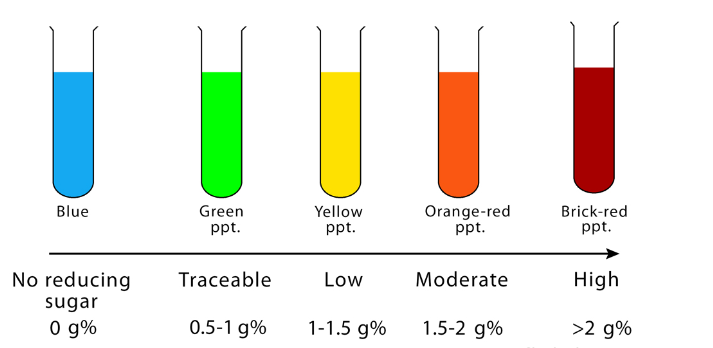
Tabulated results
| APPEARANCE OF SOLUTION | THE CONCENTRATION OF REDUCING SUGAR (%) | INTERPRETATION |
| Brick red with heavy precipitate | 2% or >2% | A large amount of reducing sugar is present. |
| Brownish orange with red precipitate | 1.5% | A moderate amount of reducing sugar is present. |
| Yellow with precipitate | 1% | A small amount of reducing sugar is present. |
| Greenish blue and cloudy | 0.5% | Traceable amount of reducing sugar is present. |
| Greenish blue with yellow precipitate | 0.25% | Traceable amount of reducing sugar is present. |
| Green with no precipitate | 0.1% | Traceable amount of reducing sugar is present. |
| Blue color or cloudy | Nil | No reducing sugar is present. |
Limitations of Benedict’s Solution
- Benedict’s Test is not specific to a particular reducing sugar. It can detect a wide range of reducing sugars, including glucose, fructose, maltose, and lactose, but it doesn’t differentiate between them.
- The test may not be sensitive enough to detect low concentrations of reducing sugars. If the reducing sugar concentration is too low, the color change may be faint or not noticeable.
- Benedict’s Test can give false-positive results in the presence of certain substances like amino acids, which also have reducing properties. This can lead to inaccurate conclusions about the presence of reducing sugars.
- The interpretation of results is somewhat subjective because it relies on comparing the color change to a color chart. Different individuals may perceive color changes differently, leading to variation in results.
- The test requires heating the sample to a specific temperature (usually boiling) for a specified duration. Variations in temperature or heating time can affect the results.
- Impurities or contaminants in the sample can interfere with the reaction, leading to inaccurate results.
- Benedict’s Test is primarily used for the detection of reducing sugars and may not be suitable for the analysis of complex mixtures or non-carbohydrate compounds.