Objective
Barfoed’s test is a biochemical test devised by the Swedish physician C.T Barfoed (1815-1899). The test is a chemical test used to differentiate between monosaccharides (simple sugars) and disaccharides, particularly between monosaccharides containing an aldehyde group and those that do not.
The main objective of Barfoed’s test is to distinguish between monosaccharides and certain disaccharides based on their ability to reduce cupric ions (Cu²⁺) in an acidic solution.
The test relies on the fact that monosaccharides, such as glucose and fructose, contain a free aldehyde or keto group that can undergo oxidation-reduction reactions, reducing cupric ions to cuprous ions (Cu⁺) in the presence of an acidic medium and heat.
Disaccharides generally lack a free aldehyde or keto group in their structure and therefore do not readily undergo this reduction reaction.
Principle
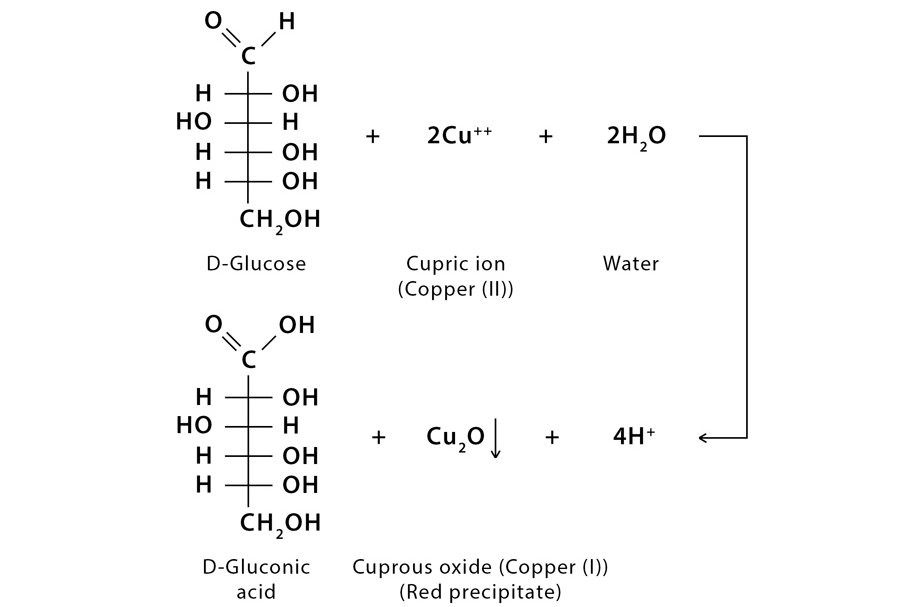
Barfoed’s test reaction is based on the reduction of cupric acetate by reducing monosaccharides and reducing disaccharides. Reduction of cupric acetate produces cuprous oxide which gives a brick red precipitate.
The reaction is conducted in a slightly acidic medium. A mixture of ethanoic (acetic) acid copper (II) acetate is added to the test solution and boiled.
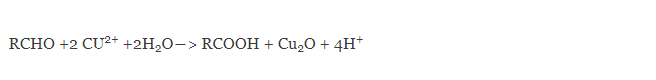
Reducing monosaccharides react with Bedford’s reagent much faster than disaccharides and produce a copious amount of red precipitate of copper (I) oxide within three minutes. Disaccharide sugars as they are weaker reducing agents, react at a slower rate and so do not form red precipitate even for 10 or 12 minutes. The reaction with disaccharides is slower because disaccharides have to get hydrolyzed first and then react with the reagent cupric acetate to produce cuprous oxide.
Reagents For Barfoed’s Test
- Copper acetate: Provides an acidic medium
- Acetic acid: Provide cupric ions
- Test solution: 5% Glucose, 5% Sucrose, 5% Maltose, 5% Lactose, 5% Starch
- Water bath
- Barfoed’s reagent: Dissolve 13.3g of copper acetate in 200 ml of distilled water and add 1.8 ml of glacial acetic acid to it.
- Dry test tubes
- Pipettes
Procedure
- Prepare the Barfoed’s reagent by dissolving 13.3g of copper acetate in 200 ml of distilled water in a clean container. Then, add 1.8 ml of glacial acetic acid to the solution and mix thoroughly. This solution will provide an acidic medium and cupric ions required for the test.
- Label the dry test tubes accordingly to identify the samples to be tested.
- Using a pipette, add approximately 2-3 ml of each test solution (5% glucose, 5% sucrose, 5% maltose, 5% lactose, and 5% starch) to separate test tubes. Ensure each test tube contains the same volume of test solution.
- Add an equal volume (2-3 ml) of Barfoed’s reagent to each test tube containing the respective test solution. Mix the contents of each test tube thoroughly by swirling gently.
- Place the test tubes in a water bath or heat them directly using a suitable heating source. Heat the test tubes gently to boiling, avoiding overheating.
- Continue heating the test tubes for about 3-5 minutes while observing any color changes. If reducing sugars (such as glucose) are present in the test solution, a reddish precipitate of cuprous oxide (Cu₂O) will form upon heating.
- After heating, remove the test tubes from the water bath or heating source and allow them to cool to room temperature.
- Observe each test tube for the presence of a reddish precipitate at the bottom or along the sides of the tube. The formation of a reddish precipitate indicates a positive result for reducing sugars, specifically monosaccharides with aldehyde groups.
- Record your observations for each test tube, noting whether a reddish precipitate is present or absent.
- Dispose of the contents of the test tubes properly according to laboratory waste disposal guidelines.
- Rinse the test tubes thoroughly with distilled water to remove any residue before reuse or storage.
Precautions:
- Handle chemicals such as copper acetate and acetic acid with care and follow proper safety procedures.
- Avoid overheating the test tubes to prevent decomposition or charring of the samples.
- Dispose of chemical waste properly according to laboratory safety protocols.
- Wear appropriate protective gear, such as gloves and goggles, when handling chemicals.
Result Interpretation
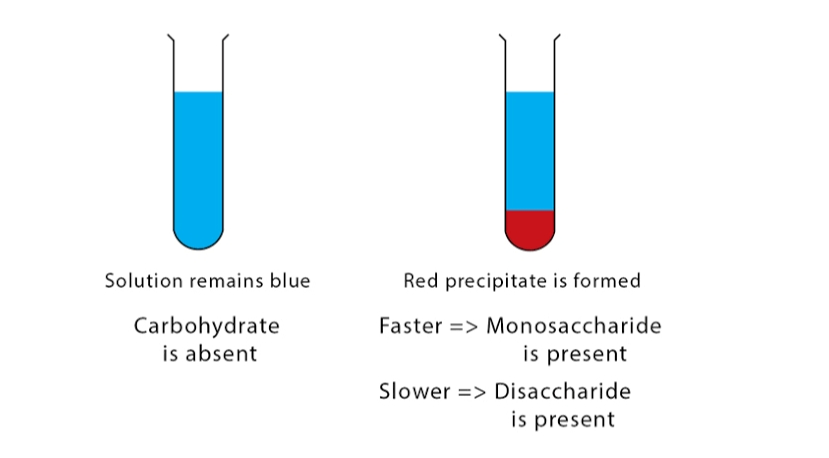
- Positive Barfoed’s Test: Brick red precipitate forms within 5 minutes of boiling in the case of monosaccharide while for disaccharides, the precipitate forms between 7-12 minutes.
- Negative Barfoed’s Test: Absence of red color.
Limitations of Barfoed’s Test
- Barfoed’s test can only distinguish monosaccharides from certain disaccharides based on their reducing properties. It cannot differentiate between different types of monosaccharides or provide information about the specific structure of the carbohydrates present.
- Barfoed’s test usually detects monosaccharides containing an aldehyde group, such as glucose and fructose. However, it does not detect monosaccharides with a ketone group, such as fructose in its ketose form. Therefore, ketoses may not be identified using this test.
- Barfoed’s test relies on the reduction of cupric ions by monosaccharides. However, other reducing substances present in the sample may also produce a positive result, leading to false interpretations. For example, certain amino acids and polyhydroxy compounds can also reduce cupric ions and give a positive test result, leading to false positives.
- The interpretation of Barfoed’s test results relies on visual observation of color changes, particularly the formation of a reddish precipitate. This interpretation can be subjective and may vary depending on factors such as lighting conditions and individual perception.