Description
An alpha ray is another name for alpha radiation emitted in the type of radioactive decay referred to as alpha decay of a radioactive nucleus. The alpha ray consists of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus.
Like all kinds of radioactive decay, alpha decay occurs because the final state of the nucleus (the one decaying) has a lower energy than the initial one (the difference is the energy of the emitted alpha particle, both its binding energy and its kinetic energy).
Beta Ray also referred to as beta radiation, can be described as a high-energy, high-speed electron positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay.
Beta rays have a mass which is half of one thousand of the mass of a proton and carry either a single negative electron or positive positron charge. As they have a small mass and can be released with high energy, they can reach relativistic speeds (close to that of light).
Gamma ray also referred to as gamma radiation is a penetrating electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves and therefore imparts the highest photon energy.
Gamma rays are a form of electromagnetic radiation; they are similar to X-rays but only distinguished by the fact that they are emitted from an excited nucleus whereas x-rays are produced when electros strike a target or when electrons re-arrange within an atom.
Here is the difference between the particles.
| BASIS OF COMPARISON | ALPHA RAYS | BETA RAYS | GAMMA RAYS |
| Nature | High speed helium nucleus. | High speed Electrons | High speed electromagnetic radiations. |
| Distance Travelled | 2-4 cm. | 2-3 meters. | 500 meters. |
| Luminescence | Produce fluorescence and phosphorescence. | Produce phosphorescence. | Produce phosphorescence. |
| Penetration Power | Low | Moderate, 100 times more than alpha particles. | High, 100 times more than beta particles. |
| Charge | 2 Positive Charge. | 1 Negative charge. | No Charge. |
| Mass | 6.65 X 10^-27 Kg. | 5.5 X 10^-4 amu. | Negligible. |
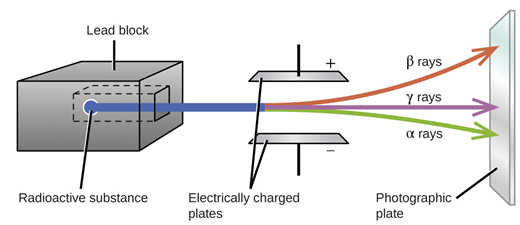
| Effect of Magnetic And Electric Field | Deflected towards the negative plate. | Deflected towards the positive plate. | Not deflected. |
| Ionizing Power | Greater than beta and Gamma rays. | Very low. | Very low. |
| Velocity | 5% of the velocity of light. | Nearly equal to the velocity of light. | Equal to the velocity of light. |
| Symbol | α | β | ϒ |